Francesco Prati, Flavio Giuseppe Biccirè
Updated on May 13, 2021
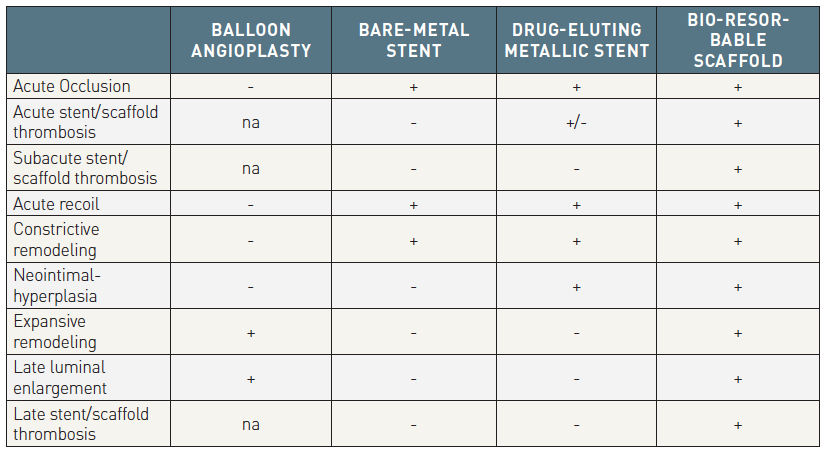
The invention of balloon angioplasty as a percutaneous treatment for obstructive coronary disease by Andreas Gruntzig in 1977 was a huge leap forward in cardiovascular medicine and will undoubtedly always be remembered as the first revolution in coronary revascularization. This technique however was plagued by multiple problems including recoil and acute vessel closure secondary to occlusive coronary dissection, which sometimes necessitated emergency coronary artery bypass surgery , , and these drawbacks drove the development of bare metal stents (BMS). Following the landmark BENESTENT and STRESS trials, which demonstrated the superiority of BMS over balloon angioplasty, BMS were promoted to be the new standard of care for percutaneous revascularization during the early 1990’s. BMS however, were still plagued by significant rates of in-stent restenosis , , and to address this, drug-eluting stents (DES) were developed by coating BMS with polymers containing anti-proliferative drugs. First generation DES significantly reduced in-stent restenosis and target lesion revascularization (TLR) compared with BMS , however remaining concerns about late and very late stent thrombosis (ST) , prompted further developments of DES, with the use of more biocompatible or biodegradable polymers, thinner struts and new antiproliferative drugs. These new iterations led to the current generation of DES that are associated with both excellent efficacy and an improved safety profile , , . New generation DES represent the latest revolution in percutaneous coronary intervention (PCI) and are considered the best currently available technology in the field, but there remains room for improvement, especially regarding very long-term clinical outcomes with these late events presumably stemming from the continued presence of a permanent metallic foreign body in the coronary arteries. The concept of a device that “does the job -i.e. provides short-term vessel support and inhibits early constrictive remodelling- and disappears” is appealing and due to their intriguing features, Bioresorbable scaffolds (BRS) have the potential to become not only another revolution, but literally the ‘holy grail’ of percutaneous revascularization . Table 1 shows the evolution of percutaneous coronary revascularization from balloon angioplasty to BMS, DES and to BRS. Current evidence does not yet support this optimistic view, however intensive research and developments are ongoing globally to advance this technology and hopefully meet expectations.
BRS have potential advantages over current metallic DES technology, including:
The word «Stent», now used in many medical disciplines, derives from the name of an English dentist born in 1807, Charles Thomas Stent. Notwithstanding this, it is Jacques Puel and Ulrich Sigwart who are credited for introducing and popularizing this term in percutaneous coronary intervention, substituting previously used terms such as intravascular prostheses or grafts. In their landmark work from 1987 , the researchers described the first use of intracoronary stents in humans, which at that time were called "Wallstents", after its inventor Hans Wallstén. Scaffold is a newer term that implies temporary arterial support and was introduced in interventional cardiology to distinguish these devices from permanent stents. For over a decade it has been widely used in peer-reviewed medical literature and is now the preferred term to describe bioresorbable coronary devices.
After the first commercial medical devices made from artificial polymers degradable in vivo (surgical sutures made from lactic acid and/or glycolic acid) were referred to as “absorbable sutures” , such devices were frequently dubbed as “bioabsorbable”, which in general reflects the disappearance of a compound into another substance. However, the term “bioabsorbable” is not appropriate, because “bioabsorption” does not necessarily mean “degradation”, and even less, “elimination of the polymer from the body”. Indeed, even if a “bioabsorbable” polymeric device was no longer visible due to degradation (“bioabsorption”), high molecular mass molecules could still be trapped between the skin and mucosa without passing through physiological barriers. Therefore, bioabsorption does not necessarily mean complete cleavage of macromolecules into small molecules that can be eliminated from the body through natural pathways, namely the kidney or lungs. To indicate this total elimination of polymers by excretion and assimilation, the concept of “bioresorption” was introduced , . Bioresorption indicates the total elimination of polymers from the body via natural routes (like kidney or lungs) by dissolution, assimilation, and excretion . The words “degradation” and “bio-degradation” are also confusing and should be restricted to cases of unknown or abiotic mechanisms (“degradation”) or cell-mediated in vivo mechanisms (“bio-degradation”).
The efforts to create BRS started approximately 30 years ago with the first experimental studies using non-biodegradable polyethylene-terephthalate braided mesh stents in porcine animal models published by our group in 1992 . In 1996, our group in collaboration with the Mayo and Cleveland Clinics reported the negative consequences of implanting a Wiktor stent coated with five different types of biodegradable polymers (Polyglycolic acid/ polylactic acid copolymer: PGLA, polycaprolactone: PCL, Polyhydroxy-butyrate/-valerate copolymer: PHBV, Polyorthoester: POE and Polyethyleneoxide/polybutylene terephthalate: PEO/PBTP), in porcine coronary arteries, with all experiencing marked inflammation leading to neointimal hyperplasia and/or thrombus formation. Subsequently, Lincoff et al. demonstrated that in a porcine model, a stent coated with high molecular weight PLLA (321kD) was well tolerated and effective, whilst a stent coated with low molecular weight PLLA (approximately 80kD) was associated with an intense inflammatory neointimal response. The authors also proved the feasibility of drug-elution from PLLA, although no suppression of neointimal hyperplasia was reported. In 1998, Yamakawa et al. reported that in the porcine model a fully biodegradable PLLA stent with a tyrosine kinase inhibitor efficiently suppressed proliferative stimuli caused by balloon injury. These pioneering experiments with high-molecular-weight PLLA further supported investigations in human. However, even though the idea of a BRS was present from the early days of stent development, the technology failed to develop at that stage due to the lack of an ideal polymer and the advent of metallic DES , and matured long after DES had become the gold standard for percutaneous revascularization. In 2000, the Igaki-Tamai PLLA-based scaffold became the first BRS to be implanted in humans and aliphatic polyesters have dominated the field as scaffold materials ever since, despite the compromises that had to be made in various mechanical performance properties. The first investigations of magnesium alloy-based scaffolds for cardiovascular interventions started in 2003 and the first clinical trial of magnesium-based BRS for intracoronary use was published in 2007 .
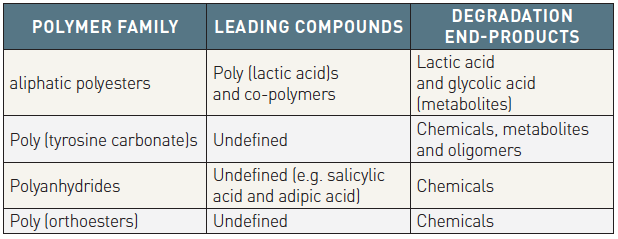
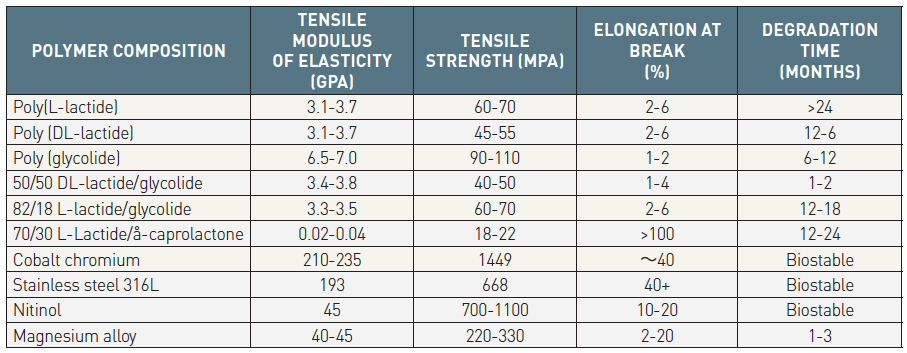
The current BRSs are composed of either a polymer or bioresorbable metal alloy. Numerous different polymers are available, each with different chemical compositions, mechanical properties and bioabsorption times (Table 2 and Table 3). The most frequently used polymer in the current generation of BRS is PLLA, which is already in widespread clinical use in resorbable sutures, soft tissue implants, orthopedic implants, and dialysis media. The key mechanical traits for candidate material for coronary indications include high elastic moduli for radial stiffness, large break strains to impart the ability to withstand deformations from the crimped to expanded states, and low yield strains to reduce the amount of recoil and overinflation necessary to achieve a target deployment. Scaffold developers have looked to increase stent strut dimensions to compensate for the mechanical shortcomings of bioresorbable materials, however increases in strut thickness lead to a proportional increase in strain levels imposed on the material.
The efforts to create BRS started approximately 30 years ago with the first experimental studies using non-biodegradable polyethylene-terephthalate braided mesh stents in porcine animal models published by our group in 1992 . In 1996, our group in collaboration with the Mayo and Cleveland Clinics reported the negative consequences of implanting a Wiktor stent coated with five different types of biodegradable polymers (Polyglycolic acid/ polylactic acid copolymer: PGLA, polycaprolactone: PCL, Polyhydroxy-butyrate/-valerate copolymer: PHBV, Polyorthoester: POE and Polyethyleneoxide/polybutylene terephthalate: PEO/PBTP), in porcine coronary arteries, with all experiencing marked inflammation leading to neointimal hyperplasia and/or thrombus formation. Subsequently, Lincoff et al. demonstrated that in a porcine model, a stent coated with high molecular weight PLLA (321kD) was well tolerated and effective, whilst a stent coated with low molecular weight PLLA (approximately 80kD) was associated with an intense inflammatory neointimal response. The authors also proved the feasibility of drug-elution from PLLA, although no suppression of neointimal hyperplasia was reported. In 1998, Yamakawa et al. reported that in the porcine model a fully biodegradable PLLA stent with a tyrosine kinase inhibitor efficiently suppressed proliferative stimuli caused by balloon injury. These pioneering experiments with high-molecular-weight PLLA further supported investigations in human. However, even though the idea of a BRS was present from the early days of stent development, the technology failed to develop at that stage due to the lack of an ideal polymer and the advent of metallic DES , and matured long after DES had become the gold standard for percutaneous revascularization. In 2000, the Igaki-Tamai PLLA-based scaffold became the first BRS to be implanted in humans and aliphatic polyesters have dominated the field as scaffold materials ever since, despite the compromises that had to be made in various mechanical performance properties. The first investigations of magnesium alloy-based scaffolds for cardiovascular interventions started in 2003 and the first clinical trial of magnesium-based BRS for intracoronary use was published in 2007 .
The current BRSs are composed of either a polymer or bioresorbable metal alloy. Numerous different polymers are available, each with different chemical compositions, mechanical properties and bioabsorption times (Table 2 and Table 3). The most frequently used polymer in the current generation of BRS is PLLA, which is already in widespread clinical use in resorbable sutures, soft tissue implants, orthopedic implants, and dialysis media. The key mechanical traits for candidate material for coronary indications include high elastic moduli for radial stiffness, large break strains to impart the ability to withstand deformations from the crimped to expanded states, and low yield strains to reduce the amount of recoil and overinflation necessary to achieve a target deployment. Scaffold developers have looked to increase stent strut dimensions to compensate for the mechanical shortcomings of bioresorbable materials, however increases in strut thickness lead to a proportional increase in strain levels imposed on the material.
The term “polymer” originates from the Greek words «πολύ» (many or much)=poly and «μέρος» (part)=mer and refers to a macro-molecule that is composed of multiple repeated sub-units. Polymers have specific characteristics that define their mechanical properties which in turn define their suitability to be used as materials for scaffold development. The key polymer characteristics, beyond their type, include molecular weight, crystallinity, and hydrophobicity, while the key mechanical properties are tensile strength, elasticity, phase behaviour and biodegradation rate. The polymer’s weight-averaged molecular weight affects its processability while the number-averaged molecular weight influences its mechanical strength and elasticity , . Crystallinity is determined by the degree by which the monomers are aligned with one another and affects both tensile strength and degradation rate; in general, higher crystallinity results in longer degradation time and higher tensile strength . Hydrophobicity, from the Greek word «ύδωρ»= hydro (water) and «φόβος»=phobia, is the property of repelling water rather than absorbing it or dissolving in it and is another factor influencing the degradation rate. Tensile strength quantifies the amount of stress that a material can endure before suffering permanent deformation. Good radial strength allows thinner struts and a lower profile, hence better deliverability. Elasticity, from the Greek word «ἐλαστός»=ductible, is defined as the ability of a body to resist a distorting/deforming influence or force and return to its original size and shape when the latter is removed. For polymers, elastic properties are quantified by Young’s or tensile modulus of elasticity which is highly dependent on temperature. In the case of PLLA, whilst increasing its crystallinity is expected to increase its strength, this comes at the expense of its elasticity , limiting the amount of expansion that a polymer scaffold can endure during deployment without fracturing. The phase behaviour of polymers is defined by their glass transition temperature (Tg) (=the temperature that the polymer starts to display rubbery behaviour) and melting point temperature (Tm).
In the PLA family of polymers, molecular weight degradation occurs in vivo predominantly through hydrolysis, which is a bimolecular nucleophilic substitution reaction that can be catalysed by the presence of either acids or bases. The schematic shown in Figure 1A describes the hydrolysis reaction in which water catalyses a chain scission event at an ester bond.
Poly(L-lactide) is a semi-crystalline polymer, meaning that it is typically comprised of a mixture of ordered densely packed crystalline phase, and disordered less dense amorphous phase (Figure 1B). Individual polymer chains frequently span both phases; consequently, domains of crystalline polymer are linked to one another by amorphous tie chains (Figure 1B). Since the amorphous regions are less dense, water penetrates it more readily than the crystalline regions.
For objects whose smallest dimension is O (100 µm), hydrolysis byproducts cannot readily diffuse out of the object and therefore bulk degradation controls the process. The object degrades more or less from the inside out. In this case, the third order kinetics theory of Pitt et al. predicts that the hydrolysis rate depends on the concentration of ester bonds, water, and carboxylic acid end groups, the latter of which are generated by each hydrolysis reaction , , . This so-called autocatalytic model for aliphatic polyesters like PLA is based upon the third-order rate equation given by:
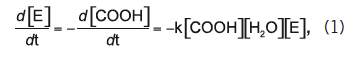
where [E], [COOH], and [H2O] represent the concentrations of ester bonds, carboxylic acid end groups, and water, respectively, and k is the hydrolytic degradation rate constant. Assuming that the concentrations of ester bonds and water are approximately constant throughout the degradation process and the concentration of carboxylic acid end groups is inversely proportional to the number-average molecular weight (Mn) of the polymer (i.e., [COOH] = 1/Mn), Weir and co-workers showed that: ,
![]()
where Mn(t) is the number-average molecular weight after degradation time t, and Mn(0) is the number-average molecular weight at time t=0 (prior to degradation). The assumptions inherent to the model are reasonable if mass loss does not occur, since mass loss would affect the concentrations of water and carboxylic end groups in the sample. Eq. (2) may be rewritten as:
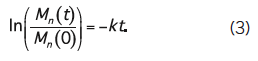
By representing data for Mn(t)/Mn(0) versus t on a log-linear plot, one may infer the hydrolytic degradation rate from the slopes of the line connecting the points (Figure 2A).
From a chemical standpoint, it is considered that resorbable implants undergo five stages which are not discrete and can overlap , . The first stage is polymer hydration; after implantation of a polymeric resorbable device, water from the environment diffuses into the material. In general PLA is relatively hydrophobic, and increasingly so as the molecular weight of the polymer chain increases. The chain ends, however, are hydrophilic due to the carboxylic acid end group. Importantly, chain ends cannot participate in a crystal lattice; therefore, the hydrophilic chain ends are relegated to the amorphous phase, rendering it more hydrophilic than the bulk. The second stage is characterized by random chain scission by hydrolysis (Figure 1A), which leads to a reduction in molecular weight. In the third stage, as hydrolysis continues, the strength of the material erodes due to the cleaving of amorphous tie chains linking the crystalline regions (Figure 2B). At this time, structural discontinuities are normal and entirely expected since the device is designed to degrade and be physiologically processed over time. In the fourth stage, polymer chains have been hydrolyzed to sufficiently short lengths that they are increasingly hydrophilic (and hence, soluble in an aqueous environment) and able to diffuse out of the device (loss of mass, Figure 2C). The fourth phase is assimilation or dissolution of the monomer. Phagocytes can assimilate small particles and lead to soluble monomeric anions. Lastly, in the fifth stage, the soluble oligomeric PLA molecules that have diffused out of the device ultimately hydrolyze to the lactic acid monomer, which deprotonates readily to lactate. Lactate is in turn converted to pyruvate, which eventually enters the Krebs Cycle and is further converted into carbon dioxide and water. These final products are excreted from the body through the kidneys or lungs, which results in complete bioresorption of the implant .
Due to the described properties of semi-crystalline polymers, they are used predominantly for the purposes of mechanical support (i.e. the scaffold backbone), whereas amorphous polymers allow a more uniform dispersion of drug, and are therefore preferred for controlled drug release systems (e.g. coating of the ABSORB BRS).
The structural changes during the bioresorption process of 4 PLLA-based products are shown in Figure 3.
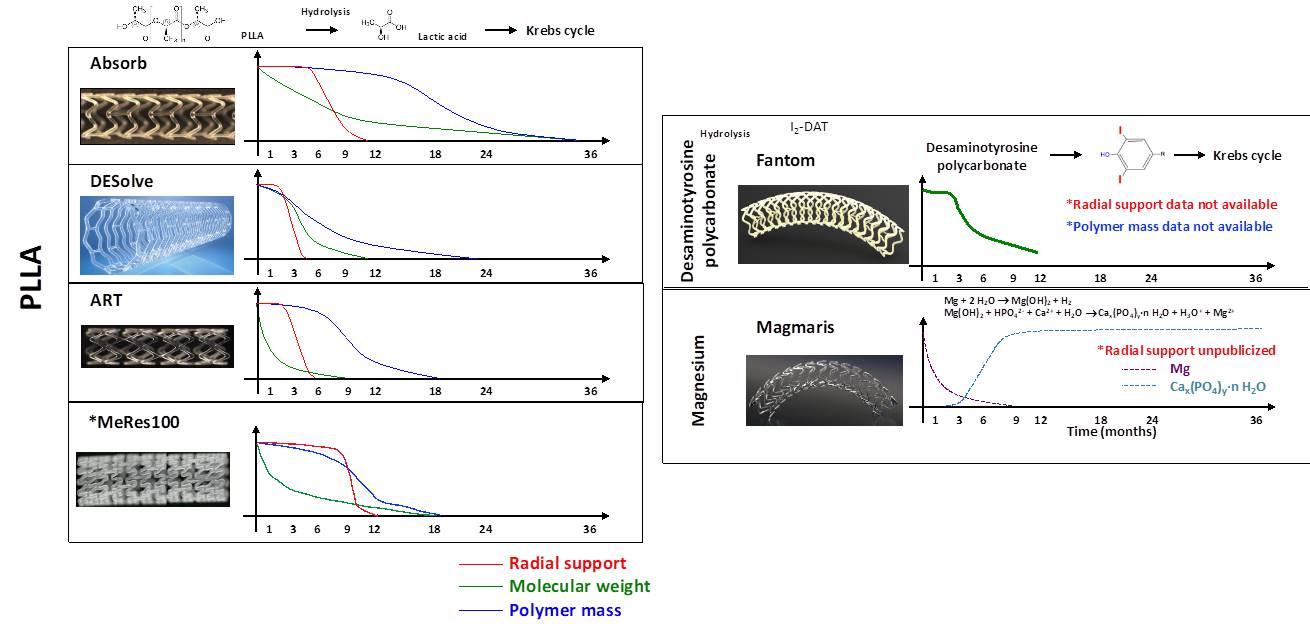
Biodegradation process of CE-mark approved BRS. Current BRS are composed of either a polymer or a bioresorbable metallic alloy. Numerous different polymers are available, each with different chemical composition, mechanical properties, and consequently bioabsorption times. BRS: bioresorbable scaffold, PLLA: poly-L-lactic acid, DAT: desaminotyrosine.
Tyrosine-derived polycarbonates are a class of polymers that contain amino acid-like backbones connected by carbonate bonds, resulting in strong mechanical properties with preservation of the biocompatibility of their degradation products (Figure 3). Their resorption pathway is similar to PLLA.
Polyanhydrides have the unique property that the degradation of the anhydride bonds is dependent on the chemistry of the polymer’s backbone, thus allowing precision pay-load release, but with poor mechanical properties.
In its pure form, magnesium has low strength and rapid biodegradation. Therefore, in BRS it is used in alloy forms, with a variety of other elements added through a range of methods to adjust its mechanical and physical properties. Precipitation-hardened magnesium alloys have a high strength-to-weight ratio , enhanced biocompatibility and low thrombogenicity. However, overall magnesium-based scaffolds have physical properties in-between classical metallic stents and polymeric scaffolds and suffer from very low radio-opacity and elasticity. This leads to difficulty in forming mesh-like tubular scaffolds and susceptibility to strut fractures when over-expanded, which is further aggravated by susceptibility to localized corrosion. Magnesium bioresorption occurs via corrosion, which means the degradation of metals by chemical surface reactions with aggressive components of the environment. The degradation process takes place in two steps (Figure 3), with the first the anodic reaction of the magnesium alloy in water, resulting in magnesium hydroxide, and the second, the conversion of magnesium hydroxide to magnesium phosphate and subsequent replacement by amorphous calcium phosphate. Magnesium is removed by diffusion from the amorphous matrix and is absorbed by the body. The by-product in the vessel is hydroxyapatite, which is eventually digested by macrophages.
Iron has good mechanical properties and radiopacity which make it another attractive candidate for BRS fabrication, however, it’s very slow degradation rate, interference with MRI imaging and need for special production and storage conditions make its use problematic. The process of iron bioresorption involves the oxidation of ferrous ion to ferric ion or interaction with nearby cells. A variety of methods have been described to accelerate this resorption process and adjust the mechanical strength of iron-based BRS alloys. The IBS (Lifetech Scientific, China) ultrathin (53 μm), sirolimus-eluting scaffold composed of nitrided iron with a PDLLA coating is the most mature sample in this category, however this is still at a preclinical level of testing .
A number of manufacturing companies developed scaffolds that after favorable outcomes in preclinical testing transitioned to clinical studies (Table 4), with the six CE marked devices: ABSORB (Abott), Magmaris (Biotronik), Fantom (REVA Medical), DESolve (Elixir Medical), MeRes100 (Meril Life Sciences), and ART Pure (Arterial Remodeling Technologies).
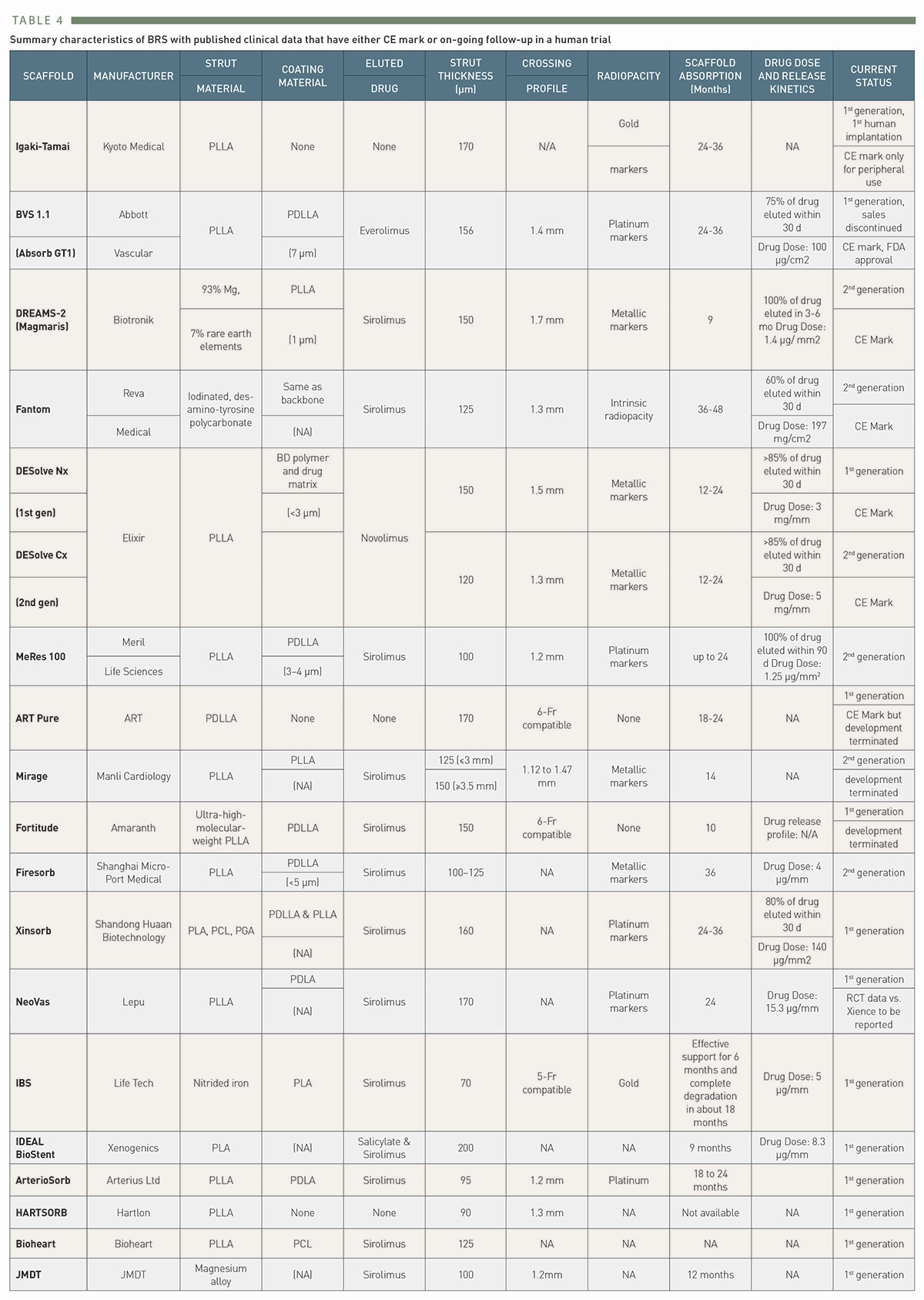
Summary characteristics of BRS with published clinical data that have either CE mark or on-going follow-up in a human trial
The Igaki-Tamai PLLA coronary stent was the first fully bioresorbable stent to be implanted in humans with complete degradation taking 18-24 months. The stent had a helical zigzag design, which differed from previous knitted patterns (Figure 4). This resulted in less vessel wall injury during implantation and therefore less initial thrombus formation and reduced intimal hyperplasia. The stent was mounted on a standard angioplasty balloon and was both thermal self-expanding and balloon expandable. Self-expansion occurred in response to heating the PLLA, which was achieved by using heated contrast (up to 70 °C) to inflate the delivery balloon. The first in man (FIM) study of the Igaki-Tamai stent (15 patients, 19 lesions, 25 stents), demonstrated no major adverse cardiovascular events (MACE) or ST within 30 days, and one repeat PCI at 6 months follow-up . Encouragingly, the loss index (late loss/acute gain) was 0.48mm, which was comparable to BMS, and demonstrated for the first time that BRS did not induce an excess of intimal hyperplasia. The long term-follow up results (n=50) were fully reported in 2012 and demonstrated TLR rates of 28% with 1 cardiac death (CD), 4 myocardial infarctions (MI) and 2 definite scaffold thromboses (ScT) at 10 years . Despite this good safety profile and the potential for further improvement with the addition of an anti-proliferative drug, the device fell out of favor for use in coronary arteries, because of concerns about the intracoronary use of heated contrast and is now only of historic value. However, it has achieved and retains a CE mark for peripheral use (REMEDY stent) .
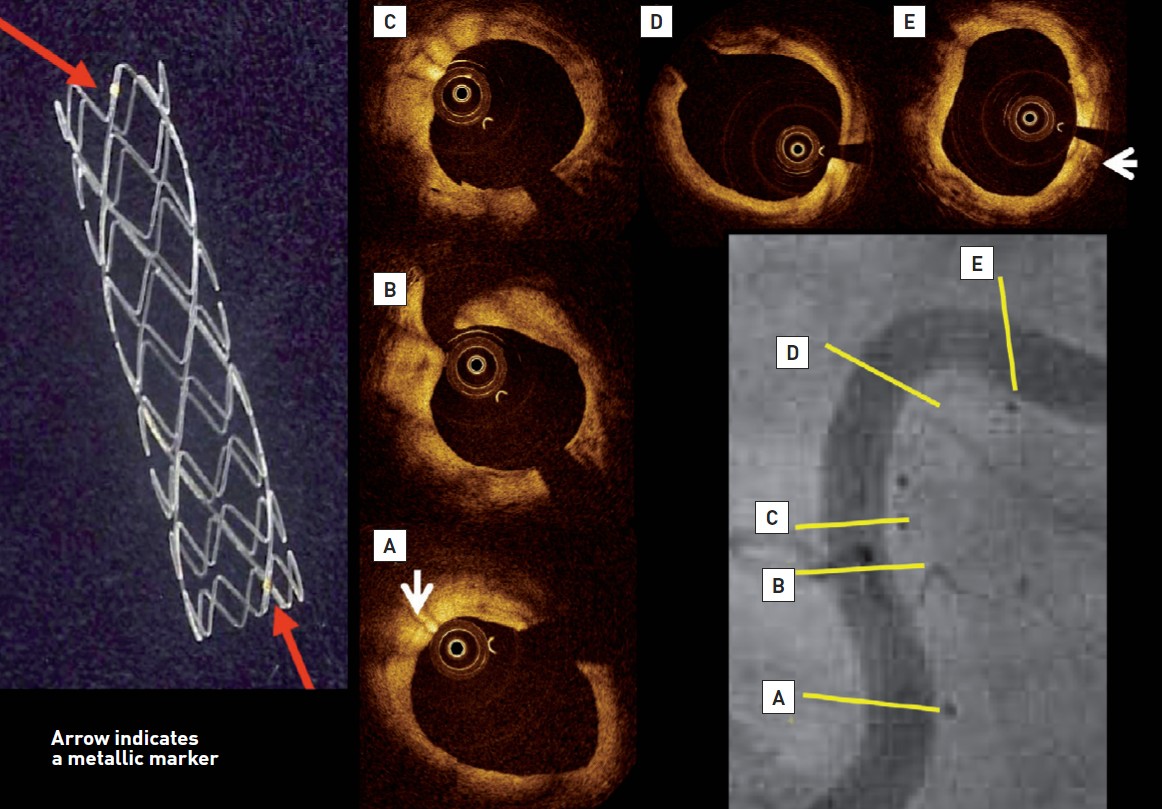
The left panel demonstrates macroscopic view of the Igaki-Tamai PLLA stent, which has zig-zag helical coils with straight bridges and golden markers at both ends of the strut. The coronary angiography performed 10 years after implantation showed the patency and curved trajectory of the previously stented lesion in the proximal right coronary artery (Lower right panel). Stent markers of the overlapping IGAKI-TAMAI stents are clearly visible in the vessel wall. On OCT, endoluminal surface of the stent was smooth and struts were no longer visible, whereas radio-opaque markers remained detectable (white arrows).
The ABSORB BRS has a PLLA-based backbone with a PDLLA coating. The later contains everolimus with a coating to drug ratio of 1:1 and controls its release in a purely diffusion-related fashion . The first-generation, 1.0, was tested in the FIM ABSORB A trial (n = 30), demonstrating very late lumen enlargement (from 6 months to 2 years), restoration of vasomotion and endothelial function at 2 years , , with a MACE rate of 3.4% and no ScT at 5 years . In the second-generation device, BVS 1.1, the design of the hoops and the manufacturing process of the polymer were modified to achieve more uniform and increased radial support, preservation of mechanical integrity for a longer period , better stent security and the convenience of storage at room temperature. The BVS revision 1.1 was tested in the FIM ABSORB cohort B trial, enrolling 101 patients who were split in two parts, undergoing multimodality invasive imaging at different follow-up periods, ranging from 6 months to 5 years after the index procedure. In the entire cohort B, the rates of MACE and ischemia driven (ID) TLR were 11% and 8% respectively at 5 years, with no cases of ScT . Optical coherence tomography (OCT) findings in the ABSORB Cohort B Trial demonstrated that acute scaffold disruption was a rare iatrogenic phenomenon that had been anecdotally associated with anginal symptoms, whereas late strut discontinuity was observed in approximately 40% of patients and could be viewed as a serendipitous OCT finding of the normal bioresorption process without clinical implications .
After the encouraging results of ABSORB B, several large registries were initiated , , to evaluate results in real world patients, but most importantly, a series of randomized controlled trials (RCTs) comparing the Absorb BRS to the Xience (Abbott Vascular) CoCr-based everolimus-eluting stent (EES) were also conducted. In general, these trials included relatively simple lesions –i.e. excluded moderately or heavily calcified or thrombotic lesions and excessive vessel tortuosity- while intravascular imaging guidance was used in only 23.9% of the BRS-treated patients with no collection of the manner in which it was applied to guide device implantation . The latest available results of these trials are presented in Table 5 including 5-year outcomes from ABSORB II, ABSORB III, ABSORB Japan, ABSORB China, and AIDA.
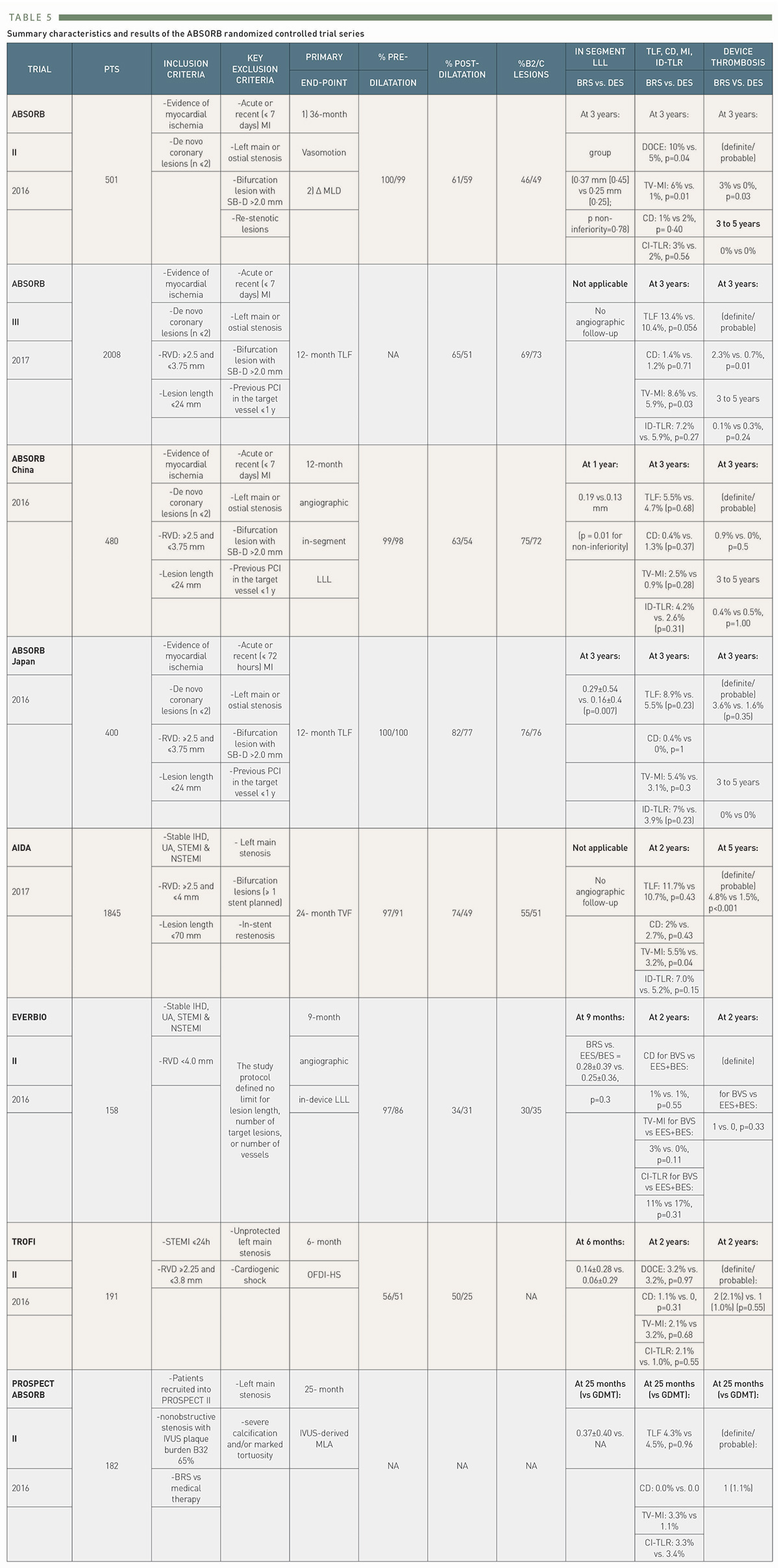
Summary characteristics and results of the ABSORB randomized controlled trial series
A meta-analysis of four of these ABSORB BVS trials (ABSORB II, III, Japan, and China) demonstrated that although rates of adverse ischemic events cumulative through 5 years were increased when compared with the EES group (n=3884), the period of excess risk ended at 3 years (, Figure 5). Hence if new-generation BRSs can demonstrate comparable results with metallic DES during their active biosorption phase, they may prove to be acceptable alternatives to metallic DESs.
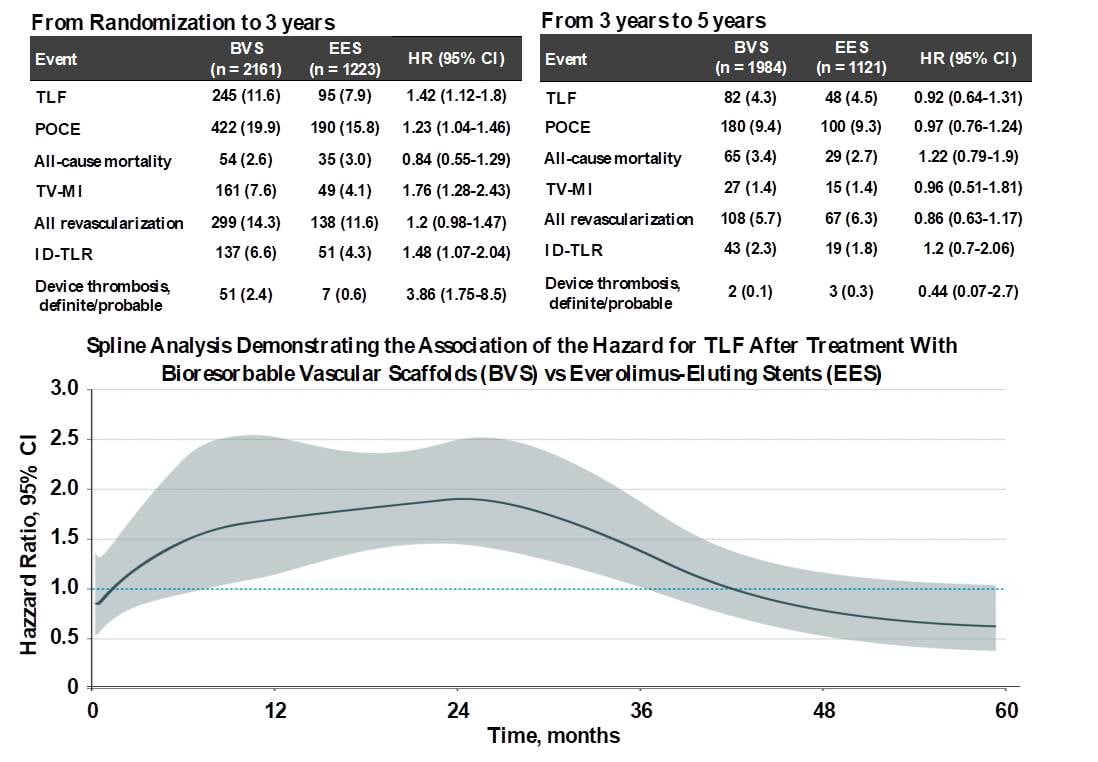
Clinical outcomes between 0 to 3 years and 3 to 5 years. Between 0 and 3 years, TLF occurred in 11.6% of 2161 BVS-treated patients vs 7.9% of 1223 EES-treated patients (HR, 1.42; 95% CI, 1.12-1.80), whereas between 3 and 5 years, TLF occurred in 4.3% of 1984 at-risk BVS-treated patients vs 4.5% of 1121 at-risk EES- treated patients (HR, 0.92; 95% CI, 0.64-1.31, P for interaction = .046) (56). By spline analysis, the HR for the risk of BVS compared with EES for TLF was relatively constant at 1.5 to 2.0 between 6 and 30 months and then progressively declined. The lower bound of the 95% CI of the HR was less than 1.0 at 36 months and the HR was less than 1.0 at 42 months (below figure). (56)
ABSORB IV was a prospective, randomized (1:1, Absorb BVS to EES), single-blind, multi-center study, enrolling 2604 patients from 147 hospitals in five countries, and showed that ABSORB BVS was non-inferior to the cobalt-chromium-based Xience DES for cardiovascular outcomes in fairly simple lesions at 1 year. In this trial there were two important design changes compared with ABSORB III – all treated vessels had to have a reference vessel diameter (RVD) >2.25 mm, and a PSP technique of pre- and post-dilation and appropriate sizing was recommended routinely. Although the results for BVS were better compared with ABSORB III, results in the Xience DES arm also improved, such that event rates were still numerically higher with BVS compared to Xience DES .
The AIDA investigator-initiated trial enrolled 1845 patients who were randomised 1:1 to the Absorb BVS or Xience DES, with the primary endpoint non-inferiority for target lesion failure (TLF, a composite of CD, TV-MI or TLR) at 2 years. The trial’s data and safety monitoring board recommended early reporting of the study results due to safety concerns after a median follow-up of 707 days, by which point no significant differences in the primary end point (11.7% vs. 10.7%, p=0.43), CD or TLR were seen. However, a higher incidence of TV-MI (5.5% vs. 3.2%, p=0.04) was seen with Absorb BVS, driven by increased rates of definite or probable ScT (3.5% vs. 0.9%, p<0.001); notably no major predictors of ScT were identified . Recently, final 5-year results of the AIDA trial have demonstrated that the excess risk with Absorb BVS on late adverse events, in particular ScT, continues up to 4-years and seems to plateau thereafter .
EVERBIO II had the most liberal inclusion criteria in the ABSORB RCT family. In this single-center study, a total of 240 patients were randomized 1:1:1 to Absorb BRS, durable polymer EES and a biolimus-eluting bioabsorbable polymer DES (BES) with the primary endpoint angiographic in-device late loss at 9 months. The rates of the primary endpoint were similar between BVS and EES/BES, while a post-hoc analysis showed non-inferiority (p<0.001) of the BVS for the same end point. The study was underpowered for clinical endpoints, however notwithstanding this overall event rates were very low, whilst in the comparison between BRS and BES, device related adverse events at 2 years were significantly higher with Absorb . Five-year clinical outcomes were similar between BVS and DES patients (device-oriented endpoint 22% vs 18%, p=0.49) as were angiographic outcomes in a selected subgroup. However, definitive conclusions cannot be drawn because the EVERBIO II trial was not powered for clinical or angiographic endpoints at 5-year follow-up .
The COMPARE-ABSORB trial was an investigator-initiated, prospective study, which randomized 1,670 patients at high risk of restenosis to receive either a BVS (n=848) or an EES (n=822). Study enrolment was discontinued prematurely because of a high rate of thrombosis and TVMI in the BVS arm. TLF occurred in 43 patients (5.1%) in the BVS group and 34 patients (4.2%) in the EES group, meeting the pre-defined criteria for non-inferiority (absolute difference 0.9%, 95% CI:−1.2%-3.0%, p non-inferiority <0.001) .
The PROSPECT ABSORB trial was designed to evaluate the effects of treating vulnerable plaque with ABSORB BVS in patients with ACS who underwent successful PCI in the PROSPECT II natural history study . Near-infrared spectroscopy-intra vascular ultrasound (NIRS-IVUS) imaging was performed in all three coronary arteries to identify non-target, non-flow-limiting lesions which were then randomly assigned to two strategies: ABSORB BVS implantation plus medical treatment vs. medical treatment alone. At 25-months, angiography and NIRS-IVUS imaging follow-up in 167 out of 182 patients (91.8%) showed that the MLA at the original site increased from 3.2mm2 to 6.9mm2 in the ABSORB BVS arm, and was unchanged in patients treated with medical therapy alone (p<0.001). The primary safety endpoint of TLF occurred in 4.3% and 4.5 % of patients in the ABSORB BVS and medical treatment arms, respectively (p=0.96). After a median follow-up of 4 years, lesion related MACE rates were 4.3% in the ABSORB BVS arm and 10.7% in the medical treatment arm (p=0.12). The results from PROSPECT ABSORB indicate that PCI of vulnerable plaques can safely enlarge the lumen and change the structure of the lesion, theoretically reducing its propensity for thrombosis and progression. The favorable randomized lesion-related MACE rates observed after BVS treatment compared with medical therapy alone warrants the performance of an adequately powered randomized trial to determine whether PCI of vulnerable plaques improves patient outcomes.
BRS is considered a promising treatment option for ST-elevation MI (STEMI) patients because these patients are often young, and ruptured plaques are usually soft with a small plaque burden. TROFI II was another relatively small RCT (n=191), whose importance lies in the fact that it included only STEMI patients undergoing primary PCI who were randomized to Absorb BVS or Xience DES. The primary endpoint was the 6-month optical frequency domain imaging healing score (HS), which was based on the presence of uncovered and/or malapposed stent struts and intraluminal filling defects and was lower in the Absorb arm (Pnon-inferiority<0.001) . At 2 years, rates of all clinical secondary endpoints were similar between the two devices , however too few events occurred to allow any meaningful statistical comparisons or clinical correlations. The five-year follow-up of the Prague 19 study (n=117) enrolling STEMI patients treated with Absorb BVS reported a composite primary endpoint (death, MI or target vessel revascularization) of 12.6% with overall mortality 6.3%, and 2 ScT during the early phase .
The Absorb BRS, the most studied device among all BRS, suffered a major setback following the negative results of the ABSORB trials, with the manufacturer deciding in 2017 to halt sales worldwide. The potential device-specific factors related to increased events were: 1) a less strong mechanical property, 2) a larger strut thickness and footprint which can predispose to under-expansion/protrusion of struts, eventually resulting in increased thrombogenicity, and 3) bioresorption time and failure of strut encapsulation before dismantling. The 2018 ESC/EACTS Guidelines on myocardial revascularisation do not currently recommended BRS for clinical use outside of clinical studies (Class III, Level C) ; however, an unanswered question is whether the failures of Absorb are applicable to other BRSs due to the following facts: mechanical properties vary with each bioresorbable material (even amongst PLLA by post-processing of the polymer); new devices have thinner struts and/or a smaller footprint, whilst the thrombogenicities of various materials are different (e.g., magnesium scaffold) ; each device has a unique biodegradation profile (bioresorption duration ranging from one to three years). Moreover, the early clinical data are inadequate to respond fully to the class effect theory in view of the small sample sizes, inclusion of simple lesions, and the learning curve. Further clinical evidence is therefore necessary to confirm the absence (or presence) of a class effect and currently approximately 34 BRSs from 22 companies are under development.
Magnesium (Mg) is the fourth commonest cation within the human body; the total body content is ~20g, with 350mg required daily. A high dose infusion of Mg can cause vasodilatation; the promotion and recruitment of collaterals during ischaemia; and can function as a direct inhibitor of stent thrombosis. AMS 1 (BIOTRONIK, Berlin, Germany) was the initial bare version with a strut thickness of 165 microns and was tested in the PROGRESS AMS FIM in 63 patients showing disappointing TLR rates of 45% at 1 year, despite the absence of ST, leading to the redesign of this device. The second iteration, AMS 2 (DREAMS 1), used a refined, WE43 alloy (93% magnesium and 7% rare earth elements) with slower bioresorption time (9-12 months), higher radial strength, 125 microns struts with rectangular shape and paclitaxel elution for 3 months. It was tested in the BIOSOLVE I study, which demonstrated angiographic in-stent LLL of 0.52±0.49mm at 1-year, while at 3 years TLR rates reached 6.6%, with no cases of CD, TV-MI or ScT .
A newer iteration (2nd generation DREAMS, marketed as Magmaris) has been made of the same alloy and design but with a strut thickness of 150 µm. This device elutes sirolimus instead of paclitaxel, has tantalum radiopaque markers at both ends and a modified, electropolished strut cross-sectional profile. Waksman et al. reported the device design and preclinical evaluation in a porcine coronary model (69, Figure 6). Preclinical results suggest that the Magmaris has a favourable safety profile with advanced healing relative to benchmark, low acute thrombogenicity, and absence of excessive lumen loss up to two years . Magmaris was evaluated in the international multicenter FIM BIOSOLVE II trial (n=123), which reported an in-scaffold LLL of 0.27±0.37mm at 6 months (primary endpoint) and 0.39±0.27 mm at 12-months follow-up. TLF -a composite of CD, TV-MI, clinically indicated TLR (CI-TLR) and CABG- occurred in 4 patients (3%), consisting of 1 CD, 1 TV-MI, and 2 CI-TLR . In a pooled population of the BIOSOLVE-II and BIOSOLVE-III trials (184 patients), the two-year target lesion failure rate was 5.9% without any definite/probable scaffold ScT at the early or late/very late phases . Serial intravascular imaging by IVUS and OCT demonstrated stable lumen dimensions beyond 12 months (, , Figure 7, Figure 8). After CE mark approval, the BIOSOLVE-IV study (NCT04025788) was initiated to validate the safety and efficacy of the Magmaris device in routine clinical practice with scheduled follow-up at 6 and 12-months, and then annually until 5 years. Recently, the 2-year follow-up results showed a TLF rate of 6.6%, with a good safety profile, no increase in ScT between 12- and 24-month follow-up and a CD rate of 0.5% (presented at the EuroPCR 2021). The BIOMAG-I trial, which is the FIM trial of the next generation magnesium scaffold, DREAMS 3G, is ongoing (NCT04157153).
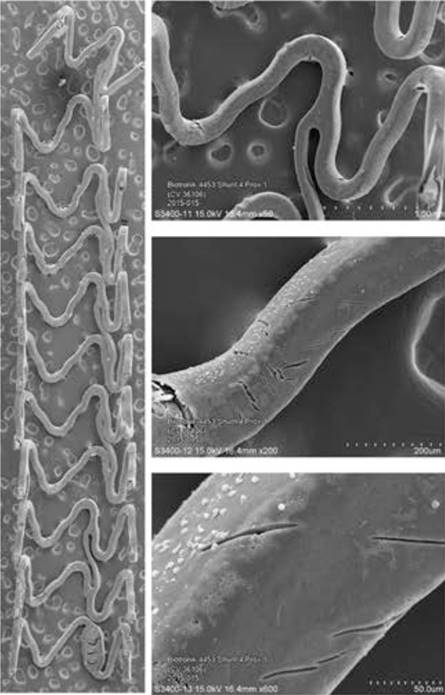
Panel A shows scanning electron microscopy images of Magmaris. Representative low- and incremental high-power scanning electron microscopy images of Magmaris after a 1-hour shunt run in the porcine ex vivo arteriovenous shunt model. Stitched low-power montage images (×15 magnification) of the entire luminal surface were then assembled into a single image. Higher-power photographs of regions of interest were also taken at incremental magnifications of (×50, ×200, and ×600). Panel B and C show scanning electron microscopy images of Magmaris assessing thrombus deposition and inflammatory cell adherence. Representative scanning electron microscopy images (×200 and ×600 magnification [above and below, respectively]) of Magmaris after a 1-hour shunt run in the porcine ex vivo arteriovenous shunt model (69).
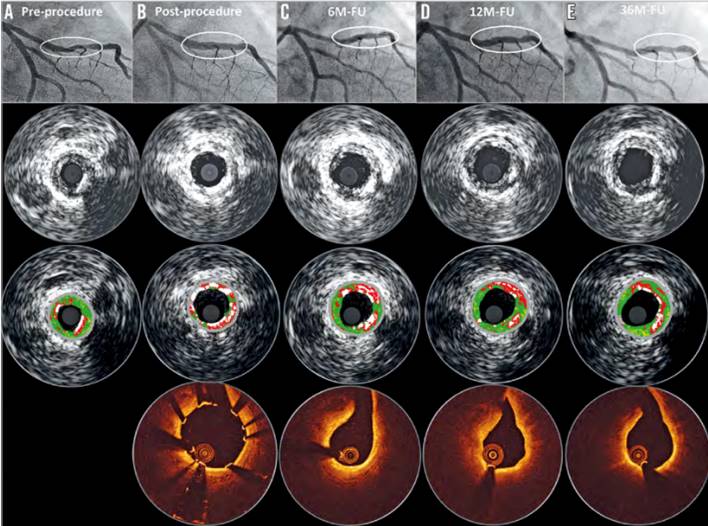
Serial quantitative angiographic, intravascular ultrasound and OCT results of a patient with DREAMS 2G implantation. A) A lesion in the mid left anterior descending (type B2, length 14 mm, diameter 2.8 mm, diameter stenosis 80% by visual estimate). Predilatation was conducted with a 3.0×12 mm semi-compliant balloon. Thereafter, a DREAMS 2G scaffold (3.0×20 mm) was implanted. Post-dilatation was performed with a 3.0×12 mm semi-compliant balloon. B) Post-procedure OCT and intravascular ultrasound demonstrate a good conformability to the vessel and a metallic appearance of DREAMS 2G. C) At six months, the degradation of the scaffold is detectable, and the struts covering the side branch have disappeared. D) At 12 months, OCT shows homogeneous neointima formation. E) Preservation of the lumen without any restenosis at 36 months (74, 75).
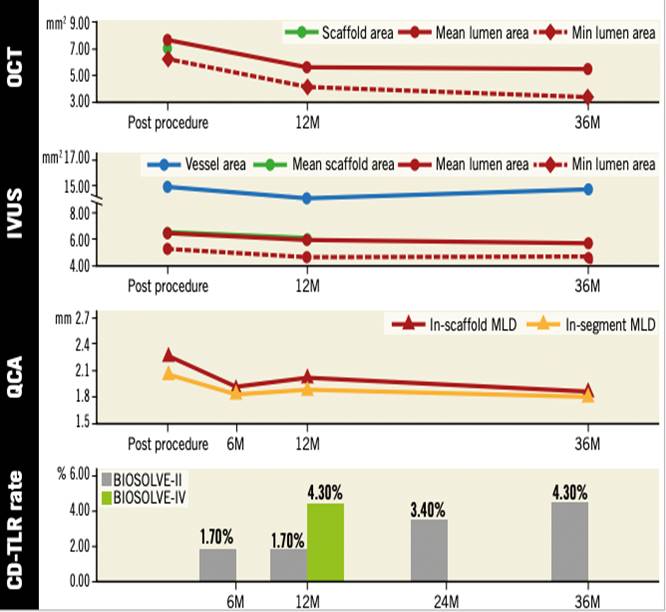
Serial change on OCT, IVUS, and QCA after implantation of Magmaris. From one to three years, a mild increase of in-stent and in-segment late lumen loss (0.11±0.28 mm and 0.13±0.30 mm) was documented, resulting in a three-year in-device and in-segment minimal lumen diameter of 1.90±0.43 mm and 1.87±0.43 mm, respectively. Serial intravascular imaging by IVUS and OCT demonstrated stable lumen dimensions beyond 12 months (74). QCA data were based on non-paired analysis, whereas OCT and IVUS data were from paired analysis. BIOSOLVE-IV is an international single-arm registry which will include 2,054 patients. At one year, the TLF rate was 4.3%, driven by clinically indicated target lesion revascularisation (74, 75).
Recently, the MAGSTEMI trial comparing MgBRS to SES in STEMI patients reported 3-year results and showed a higher rate of the device oriented composite endpoint (DoCE), a composite of CD, TV-MI, and CI-TLR with MgBRS compared to SES (16.2% vs 5.3%; p=0.030), with events driven by TLR and clustered within the first year . OCT study showed both MgBRS and SES exhibited a low degree of neointima healing, but lumen dimensions were smaller with MgBRS at one year (, Figure 9). Although the advanced bioresorption state of MgBRS hampers the assessment of scaffold collapse, this seems to be the main mechanism of restenosis. Future generations of the MgBRS ultimately need to increase and prolong radial strength to reduce the risk of restenosis.
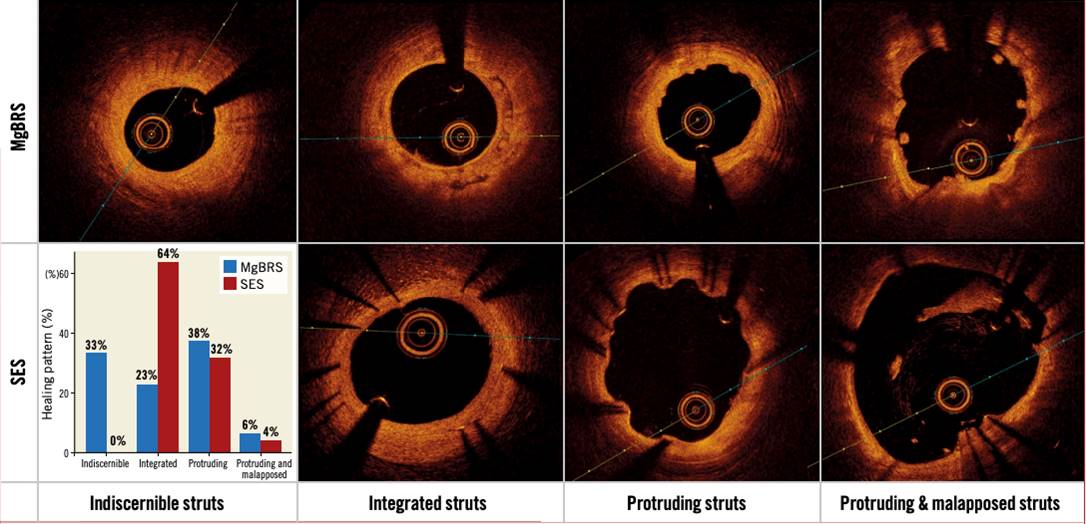
Healing and bioresorption pattern from the randomised MAGSTEMI trial. Ninety-five consecutive patients from the randomised MAGSTEMI trial (MgBRS=48, SES=47) underwent OCT imaging at one year. Healing and bioresorption pattern were categorised into four groups: 1) indiscernible struts were observed in 33.3% versus 0% of patients (p<0.001); 2) struts integrated into the vessel wall in 22.9% versus 63.8% (p<0.001); 3) protruding struts in 37.5% versus 31.9% (p=0.568); and 4) protruding and malapposed struts in 6.3% versus 4.3% (p=0.663), respectively (77).
Regarding vasomotion after implantation of MgBRS, there was no statistical significant change in vasomotion between 6 and 12 months in BIOSOLVE-II trial, whereas the rate of increase after nitro-glycerine in mean lumen diameter of the in-stent/scaffold segment was significantly higher in the MgBRS arm compared to SES arm in the MAGSTEMI trial (, , Figure 10).
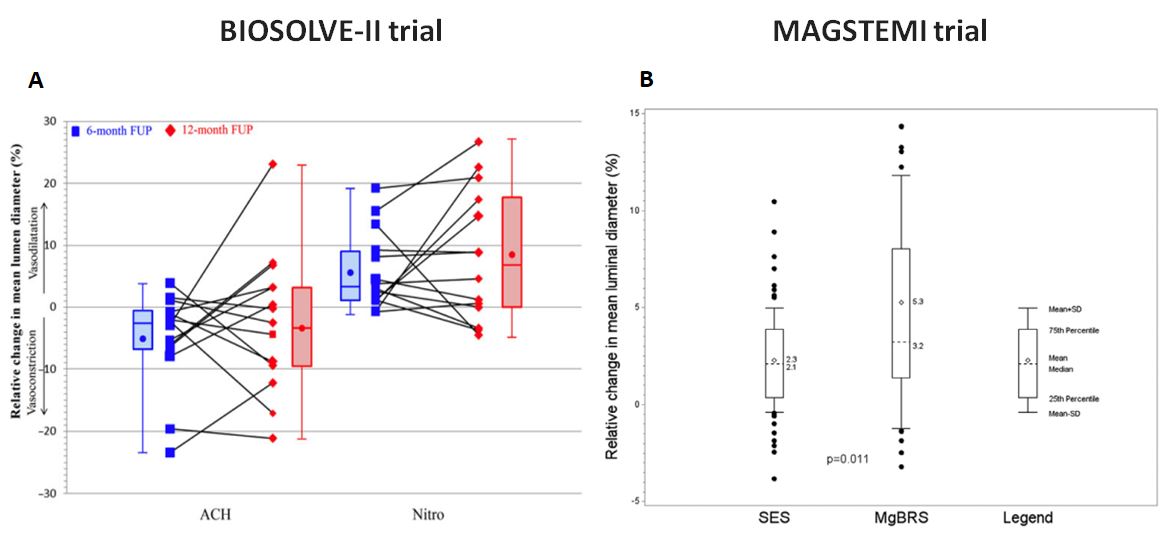
Change in vasomotion after implantation of DREAMS2G/Magmaris. Panel A shows a change in vasomotion between 6 and 12 months in BIOSOLVE-II trial (78). Percentage change in mean lumen diameter after acetylcholine and after subsequent intracoronary injection of nitroglycerine at 6 and 12 months in 14 patients. There was no statistical significant difference between 6 and 12 months (P=0.808 for acetylcholine and = 0.626 for nitroglycerine). Panel B shows an endothelial-independent vasomotor response in MAGSTEMI trial (79). At 1 year, the rate of increase after nitroglycerin in mean lumen diameter of the in-stent/scaffold segment was significantly higher in the MgBRS arm (56.5% versus 33.8%; P=0.010).
The first two iterations of the first-generation of the Reva Medical (San Diego, CA) BRS, had high rates of TLR and low rates of technical success in the RESORB FIM trial (bare form) and the RESTORE I trial (sirolimus-eluting version, ReZolve BRS), respectively . A third iteration, ReZolve BRS 2, was tested in the RESTORE II trial with results in 67 patients at 6 months showing 3 cases of MACE . However, problematic device deliverability prompted the company to feature a conventional balloon-expandable system for its next and the current iteration of the scaffold, named Fantom. The most characteristic feature of the Fantom BRS is that it contains iodine covalently bound to its desamino-tyrosine polycarbonate backbone, which makes it intrinsically radio-opaque and may decrease the need for intravascular imaging during follow-up. Furthermore, it has a wide range of expandability, while the time required for full reabsorption is significantly longer compared to PLLA based scaffolds (3 years), however more than 80% of its molecular weight loss takes place within the first year. Clinically, the Fantom BRS was initially tested in a small FIM trial and subsequently in the FANTOM II trial which included 240 patients split into two cohorts. Acute technical and procedural success was observed respectively in 95.8% and 99.1% of patients in cohort A. The 6 month angiographic and clinical primary endpoints for cohort A have been formally published , with 12-month results for the entire cohort showing a MACE rate of 4.2% with a single sub-acute ScT , and 24-month results in 125 patients showing a MACE rate of 5.6% and a single very late ScT . In addition to the latest clinical follow up, a 25-patient subset underwent angiographic imaging, with a final in-scaffold late loss of 0.25 mm, which is within the desired range of 0.2 mm to 0.4 mm. CE Mark approval for the Fantom scaffold was received in April 2017, while cohort C of the FANTOM II trial is currently enrolling patients with more complex lesions. Recently, the FANTOM STEMI trial was designed to evaluate the safety and efficacy of the device in STEMI patients, with the initial results in 10 patients showing procedural and angiographic success rates of 100% and no DoCE at 30 days . Reva has also announced a new iteration, named Fantom Encore, with a strut thickness of 95 microns.
The DESolve family of PLLA-based BRS (Elixir Medical, Sunnyvale, CA, US) includes the 1st generation device with 150 μm strut thickness and the 2nd generation device which has a strut thickness of 120 μm and elutes novolimus, an active metabolite of sirolimus. In both iterations, the scaffold is coated with a matrix of polylactide-based biodegradable polymer and antiproliferative drug, which is applied using a proprietary technique . The important features of the DESolve BRS are intrinsic self-correcting deployment properties that become operative in the event of minor strut malapposition and the ability to over-expand across a wide range of diameters without risk of strut fracture . The first iteration was initially tested as a myolimus eluting device, in the small (n=16) DESolve I FIM trial, which showed encouraging imaging and clinical results at 6 and 12 months, respectively . Subsequently, the novolimus eluting version was tested in the larger (n=126), single arm DESolve NX trial, in which the primary end point of in-stent LLL was 0.21±0.32 mm (113 patients with paired analysis) at 6 months with 98.7% strut coverage. At 5 years, rates of MACE, TV-MI, CD and CI-TLR were 9%, 1.6%, 3.3% and 4.1%, respectively; no cases of definite ScT . A single, retrospective, comparative analysis between this device and the Absorb BRS using propensity-score matching is also available, showing similar outcomes between the two devices with regards to 1-year rates of TLF, TLR, CD and definite ScT . The second iteration was tested in the relatively small (n=50), single arm DESolveCx trial, with an in-scaffold LLL at 6-months of 0.19±0.25mm and no MACE at 12 months’ follow-up . Both iterations have CE mark while the company is currently developing a 3rd generation device (Desolve NXT plus) with 120 μm strut thickness and a contoured strut design for enhanced acute performance. The prospective, randomized BIFSORB trial (NCT02973529, n=120) which compares the ABSORB BVS to the DESolve scaffold in simple bifurcation lesions with primary endpoints of safety and vessel healing at 6 months is on-going.
MeRes BRS (Meril Life Sciences, Vapi, Gujarat, India) is a PLLA-based, sirolimus-eluting BRS with a hybrid geometric structure that provides high radial strength, thin struts and tri-axial radiopaque markers. MeRes Ι FIM study (n=108) was performed in 16 medical centres in India and showed an in-scaffold LLL of 0.15±0.23 mm with “virtually complete" strut coverage (99.3%) at 6 months and very low MACE rates at 1 year (0.93%, 1 ID-TLR with no ScT). At the 3-year follow-up the cumulative MACE rate was 1.87% (two ID-TLRs) with no ScT. In-segment LLL and in-scaffold LLL between 6 months and 2 years were 0.15 ± 0.22 mm vs. 0.23 ± 0.32 mm (p=0.18) and 0.13 ± 0.22 mm vs. 0.24 ± 0.34 mm (p=0.10), respectively (, Figure 11). Based on the impressive results of the FIM trial, the company has initiated an ambitious program of further trials in non-US centres, but with worldwide participation, including MeRes-I extend and MeRes-100 China, a pivotal RCT of MeRes100 vs Xience (1:1) with a goal of enrolling 470 patients. MeRes100 received CE marking in 2019.
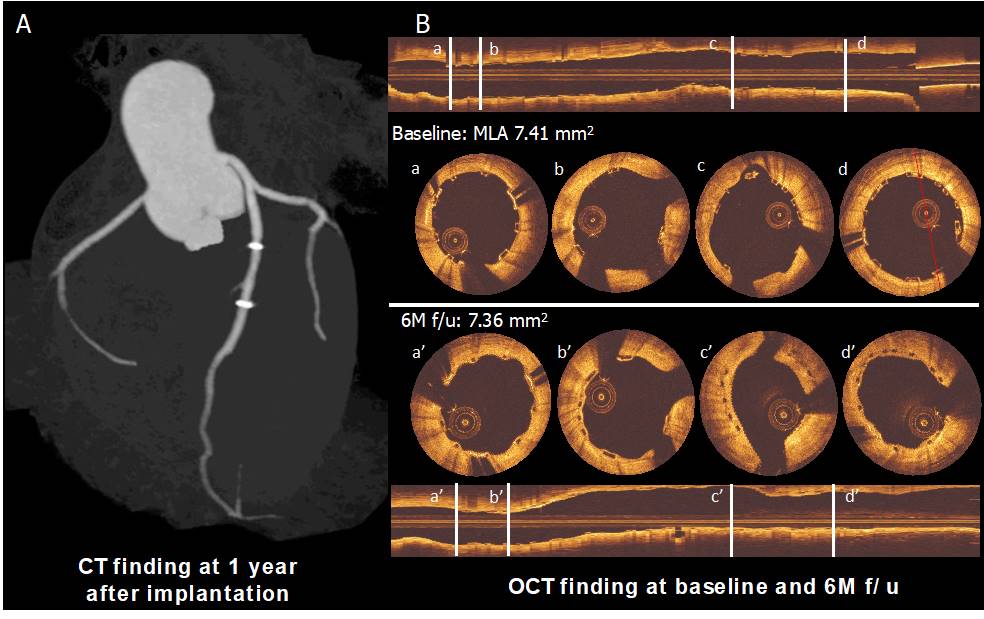
Panel A shows the computed tomography angiography (CTA) finding at 1 year after implantation. Panel B shows post-implantation and six-month follow-up OCT images. The struts are observed as preserved boxes in the baseline images while the follow-up images show open boxes because of scaffold dissolution. At six-month follow-up, the neointimal coverage of the struts is presented as the membranous bridging pattern between the open boxes (94).
The ART Pure is a PDLLA-based, non-drug eluting BRS, which was tested in the FIM ART-DIVA trial (n=30), with a primary endpoint of MACE at 6-month. No cases of CD or MI were observed, while the ID-TLR rate was 3.3% (in total 3 patients had a TLR but only one was ID) . The scaffold received CE Mark in May 2015, but was never marketed in Europe and in February 2017 Terumo announced the termination of the product’s co-development with ART.
The Amaranth scaffold family includes BRS with unique polymer production features that result in enhanced radial force and over-expansion capabilities with increased fracture resistance. The first generation device in this group is the Fortitude scaffold (150 microns), which was initially tested as a non-drug eluting version in the MEND I (n=13) trial and subsequently as a sirolimus-eluting version with encouraging results in the RENASCENT-I, MEND II and FORTITUDE (n=63) trials , , with the latter showing respective rates of TLF and ScT of 5.3% and 1.8% at 2 years. The device was then miniaturized, leading to two newer iterations, the Aptitude and Magnitude scaffolds, with strut thicknesses of 115 and 98 microns, respectively. The single arm RENASCENT-II trial enrolled 60 patients treated with the Aptitude scaffold and reported primary endpoints of safety and efficacy at 9 months . The in-scaffold LLL was 0.34±0.36 mm, whilst TLF rates were 3.4% driven by 2 cases of TV-MI, with no ScT observed. Clinical success was 98.3%, while OCT demonstrated 97% strut coverage and low rates of malapposition. Manufacturing of the Amaranth BRS scaffold is currently halted.
Mirage (Manli Cardiology Singapore) is a PLLA-based sirolimus-eluting scaffold, that incorporates a unique helix coil design for high flexibility, has relatively short bioresorption time, high scaffold dislodging force, high radial strength, can be stored at room temperature (as long as it is below 25oC) and has been shown to be “MR conditional” . It was evaluated in a single blinded clinical trial of 60 patients who were randomized to either the Mirage (n=31) or the Absorb (n=29) BRS. The primary endpoint of in-scaffold LLL at 12 months (0.48±0.49 mm for Mirage), as well as the rates of all clinical endpoints were similar between the two study groups, even though diameter stenoses 1 year after implantation on angiography and OCT were significantly higher with Mirage (, Figure 12). Manufacturing of the device is currently on hold.
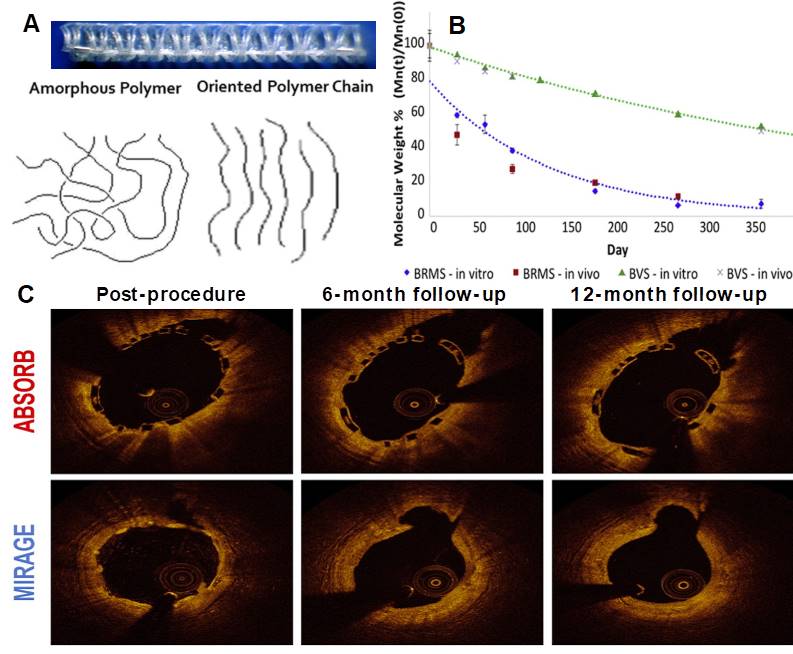
Panel A shows highly oriented polylactide polymer extruded and annealed to form a circular monofilament with preferred directional mechanical properties. Panel B shows the in vitro and in vivo degradation profile in comparison with the BVS and confirms that the Mirage polylactide is basically fully biodegraded after 14 months. Panel C shows Optical Coherence Tomographic follow-up of the Absorb and Mirage scaffolds (97).
The backbone of the IDEAL BioStent (Xenogenics Corp, Canton, Massachusetts, USA) is made of polylactide anhydride mixed with a polymer of salicylic acid with a sebacic acid linker. A salicylate coating controls the elution of sirolimus at a dose of 8.3 μg/mm while the scaffold also elutes approximately 10μg of salicylic acid. The unique design of this thick strut (200 μm) BRS is thought to provide additional anti-inflammatory properties to the anti-proliferative action of the macrocyclic lactone . This device was tested in 2009 in the FIM WHISPER study (n=11) and although the trial has not been fully reported, IVUS and OCT showed higher-than-expected intimal hyperplasia that was attributed to the rapid elution and low surface area dose of the antiproliferative drug . A new iteration has been developed with a higher dose and slower release kinetics of the drug, and also thinner struts and is currently undergoing pre-clinical evaluation.
Asian researchers have played an active role in the development of coronary artery BRS. The device characteristics and clinical results of five bioresorbable devices developed by Asian device companies under the clinical investigation phase without CE marking were summarized in Figure 13 and Figure 14.
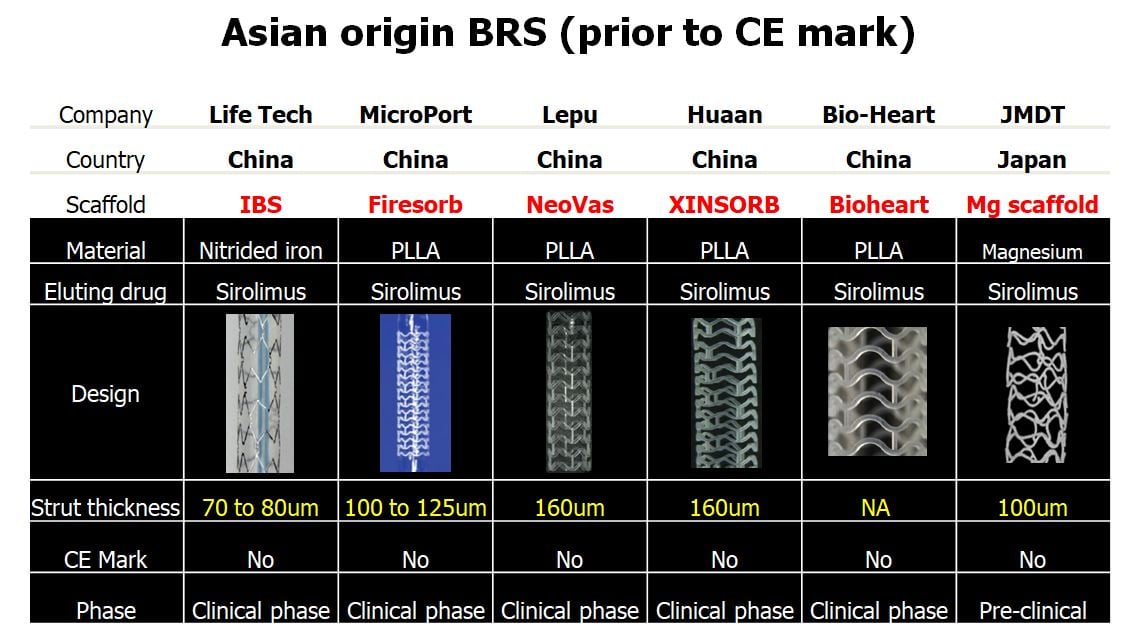
Characteristics of BRS from Asian technologies (prior to CE mark). 5 devices from China are in clinical phase and one device from Japan is in pre-clinical phase.
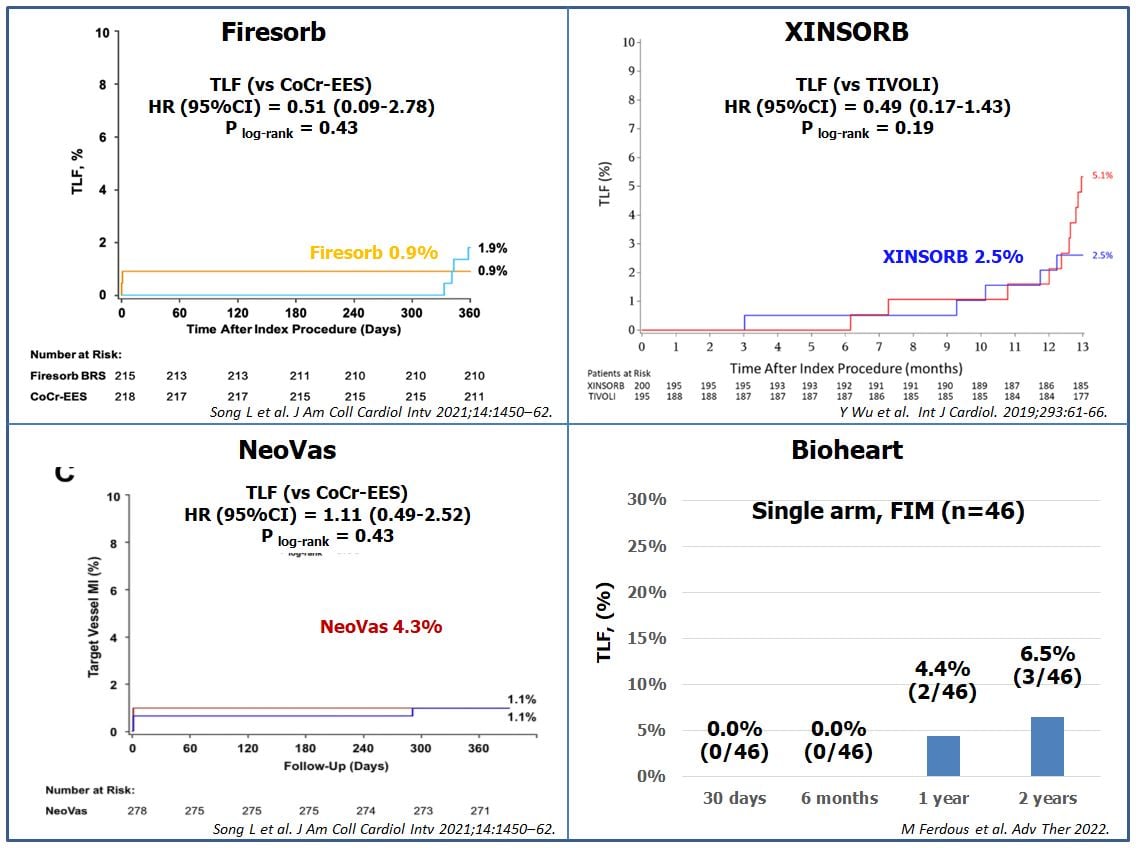
Clinical outcomes of new generation BRS from Asian technologies. Currently the results of 4 BRS companies (Firesorb, XINSORB, NeoVas, Bioheart) were published. The rate of target lesion failure of Firesorb, XINSORB, NeoVas, and Bioheart at 1 year were 0.9%, 2.5%, 4,3%, and 4,4%, respectively (105, 108, 111, 114).
Firesorb (Shanghai MicroPort Medical) is a sirolimus-eluting BRS, with a similar structure to the Absorb BRS but significantly lower strut thickness and antiproliferative drug dose (60%). It has been tested in the FIM FUTURE I trial, a single-center Chinese study in 45 patients randomly assigned to 2 cohorts (2:1), undergoing multimodality intravascular imaging at 6 months and 1 year respectively. TLF and ScT rates were 0% at 30-days (primary endpoint) and 1-year, with only 1 patient undergoing revascularization for a non-TV MI the day after the index procedure. At 6 months, in scaffold LLL was 0.15±0.11 mm and strut coverage 98.4%. At 12 months, the latter had significantly increased to 99.0%, with no other significant differences between the two cohorts in terms of imaging findings (104). In September 2017, the manufacturing company announced the enrolment of the first patient in the FUTURE II RCT which is designed to enrol 610 patients and test the Firesorb BRS against the Xience stent (NCT02890160). Recently, the 1-year results of the FUTURE II trial have reported that the thinner strut Firesorb BRS was noninferior to the CoCr-EES for the primary endpoint of 1-year angiographic in-segment LLL and the key secondary endpoint of 1-year proportion of covered struts by OCT (, Figure 14).
The Xinsorb BRS (Shandong Huaan Biotechnology Co., Ltd., Hangzhou, Zhejiang, People’s Republic of China) is a sirolimus-eluting BRS, which is stored at 4°C and has a backbone composed of a mixture of PLA, PCL and PGA and a coating of PDLLA mixed with PLL . The struts of PLLA mixed with PDLLA are coated with sirolimus (8–16 μg/mm), and have a thickness of 160 μm. The XINSORB was tested in a prospective, multi-center clinical trial (SPARTA study: NCT04501900), where patients with de novo lesions were assigned 1:1 to XINSORB and sirolimus-eluting stents (SES). Outcomes at 12-month follow-up showed a lower in-segment LLL with the XINSORB compared to SES (0.19 ± 0.32 mm vs. 0.31 ± 0.41 mm, p = 0.003), and non-inferior TLF rates (, , Figure 14). The XINSORB scaffolds and SESs showed similar efficacy and safety out to the 3-year follow-up, with low and comparable rates of TLF and device thrombosis .
This sirolimus-eluting, PLLA-based BRS with relatively thick struts (NeoVas, Lepu Medical, Beijing, China) was initially tested in an on-going follow-up FIM study of 31 patients that at 6 months reported TLF rates of 3.2%, no ScT and angiographic in-scaffold LLL of 0.26±0.32 mm with 95.7% strut coverage . A randomized trial was also performed to compare NeoVas (n = 278) to CoCr-EES (n = 282) implanted in single de novo lesions. The 12-month follow-up outcomes were similar between these two groups: LLL: 0.14 ± 0.36 mm vs. 0.11 ± 0.34 mm, p=0.62; TLF rate: 4.3% vs 3.9%, p=0.43; and probable/definite thrombosis rate: 0.4% vs. 0%, p=0.31, respectively (111, Figure 14). A pooled analysis of 1103 patients treated with NeoVas (RCT [NCT02305485]: n=278, registry [NCT02305472]: n=825) showed a 12-month TLF rate of 3.0%; a definite/probable thrombosis rate of 0.5% and a patient-oriented composite endpoint (PoCE) of all-cause death, all MI, or any revascularization rate of 5.4% . The device was approved by China Food and Drug Administration in February 2019.
IBSTM (Lifetech Scientific, Shenzhen, China) is a nitride iron-based coronary BRS. The sinusoid rings of the scaffold have 6–10 crowns which are connected circumferentially by 3–5 omega-shaped connectors to cover a diameter range of 2.0–4.0 mm and a length range of 8–38 mm. The scaffold has thin struts of 50–55μm and elutes sirolimus at 5 ug/mm . The FIM trial (NCT03509142) will enroll 65 patients, randomized to IBS V1.0 (n = 45) or IBS V1.1 (n = 20). Primary endpoints are TLF and LLL at 6 months, and patients will be followed up for 5 years after the procedure.
Bioheart BRS (Bioheart, Shanghai, China) is constructed from a PLLA backbone coated with a PDLLA layer eluting sirolimus. The strut thickness is 125 μm, with a diameter range of 3.0–3.5 mm, and a length range of 12–28 mm. The FIM trial was performed in 46 patients with de novo coronary lesions (vessel diameter: 3.0–3.75 mm, lesion length≤25 mm), with one (2.2%) definite ScT and two (4.4%) TLF occurring at 1 year follow-up (, Figure 14). Angiographic, IVUS, and OCT results confirmed the preliminary safety and efficacy at one-year follow-up, with only one patient experiencing an in-segment binary restenosis by QCA (115). Two-year clinical outcomes showed no additional ScT between 12 and 24 months. The in-scaffold LLL among the 24 patients undergoing intravascular imaging during follow-up was 0.44 ± 0.47 mm, which was primarily caused by neointimal hyperplasia rather than scaffold recoil.
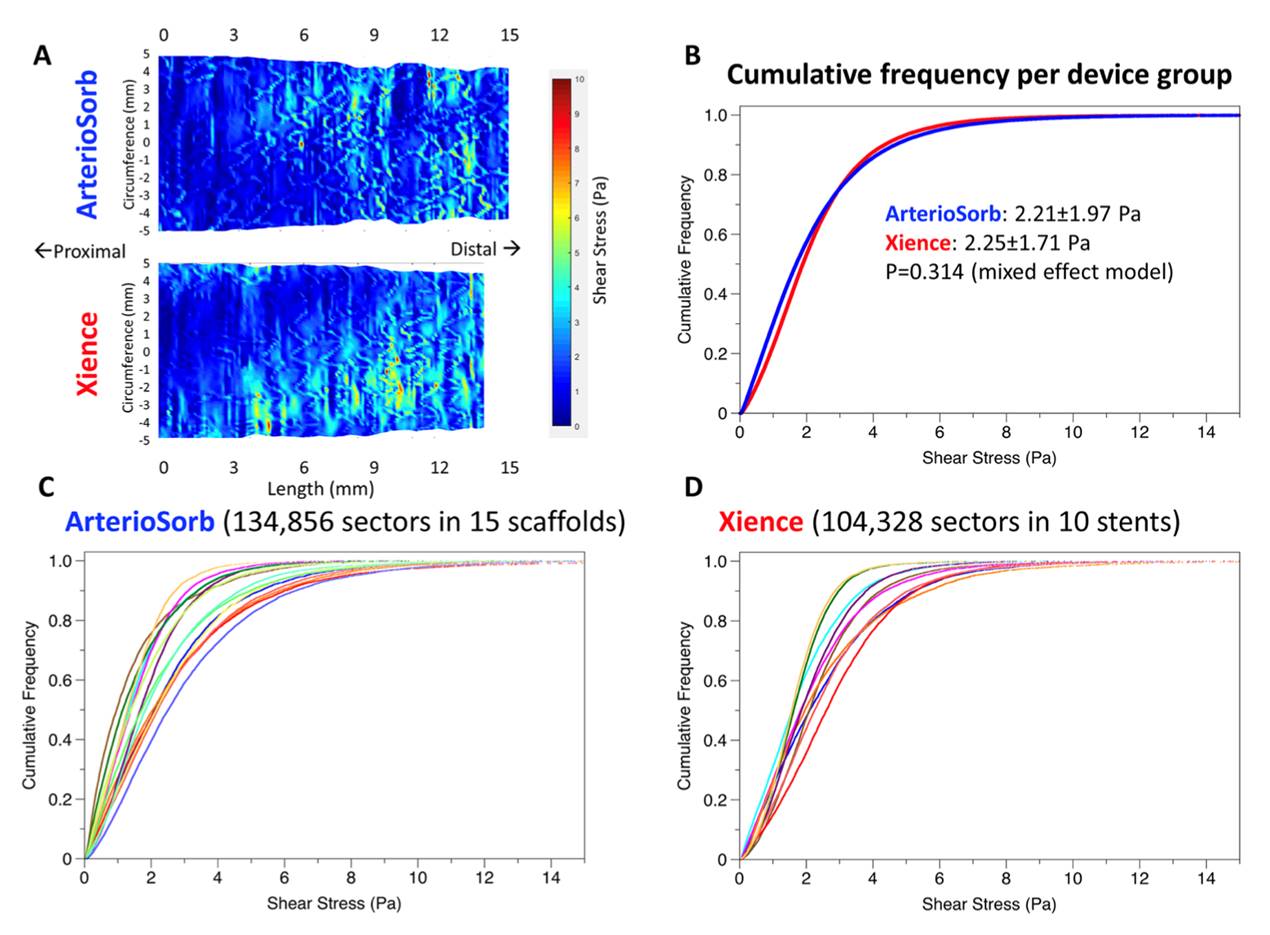
The ArterioSorb™ scaffold (Arterius Ltd, Leeds, United Kingdom) is manufactured using a patented die-drawing processing technique that results in improved radial strength and thin struts with a thickness of 95µm. The PLLA is processed further using novel technology which enables cost-effective production with high strength and stiffness and greater flexibility. The test device ArterioSorbÔ (Arterius Ltd., Leeds, UK) is composed of an 8-cell open design with smaller central cells to improve radial strength and cell connectors distributed in a spiral design. The scaffold is coated with a bioabsorbable (PDLA) polymer containing sirolimus. Bench testing has shown good radial strength following testing of an expanded ArterioSorb™, whilst acute recoil, luminal dimensions and endothelial shear stress were comparable to those of the XIENCE metallic DES (, Figure 15). The FIM trial is planned to start in 2022.
Clinical outcomes of new generation BRS from Asian technologies. Currently the results of 4 BRS companies (Firesorb, XINSORB, NeoVas, Bioheart) were published. The rate of target lesion failure of Firesorb, XINSORB, NeoVas, and Bioheart at 1 year were 0.9%, 2.5%, 4,3%, and 4,4%, respectively (105, 108, 111, 114).
HARTLON (Wilmington, Delaware, USA) has developed a novel and unique laboratory-scale process for manufacturing their BRS because the HARTSORB stent has struts comprised of a novel microporous, fibrillated polymeric material instead of being made from solid polymeric material. These microporous, fibrillated struts offer superior resistance against vessel recoil and strut fracture, whilst the stronger fibrillated material offers the potential to reduce strut thickness thereby maximizing arterial lumen capacity. The struts include very small fibrils of PLLA oriented in multiple axes between nodes of PLLA that increase the scaffold’s radial strength, whilst the void space between the fibrils increases the scaffold’s toughness, thereby reducing the potential for strut fracture during deployment or scaffold overlap. However, as fibrillated PLLA loses orientation when forming networks during heating, the company has shifted its focus to the development of ultra-high molecular weight PLLA polymers to form a scaffold with high tensile strength and ductility.
JMDT, a magnesium scaffold with 100μm thick struts, has been co-developed by the Japan Medical Device Technology company (Kumamoto, Japan) and the Fuji Light Metal company (Kumamoto, Japan). The device consists of a new, rare-earth element-free magnesium alloy , with three layers of anti-corrosion coatings and an outer layer which elutes sirolimus. In-vitro and preclinical data showed this device had improved mechanical properties and sustainable radial force during degradation.
STENTiT has a unique technological platform where support grafts are composed of polymeric microfibers, processed by electrospinning. Upon expansion, the fibrillated scaffold organizes in a circumferential orientation, reinforcing its mechanical properties, resulting in vertical compression forces comparable to metallic stents. Because of its porous structure, circulating blood cells interact with the network, triggering a natural healing response. The potential of these scaffolds was evaluated in the abdominal aorta of 16 rats, equally divided into four groups with follow-up times of 2, 4, 6, and 8 weeks. The results were recently reported, and show for the first successful minimally invasive delivery of a novel regenerative transcatheter tissue-engineered vascular graft with full patency for up to 2 months, extensive cellular infiltration with subsequent rapid endo-thelialization, favorable matrix production and remodeling with abundant neo-endoluminal elastin formation (, Figure 16). These promising short-term results in a small animal model hold future potential for various clinical indications in a variety of patients with cardiovascular disease.
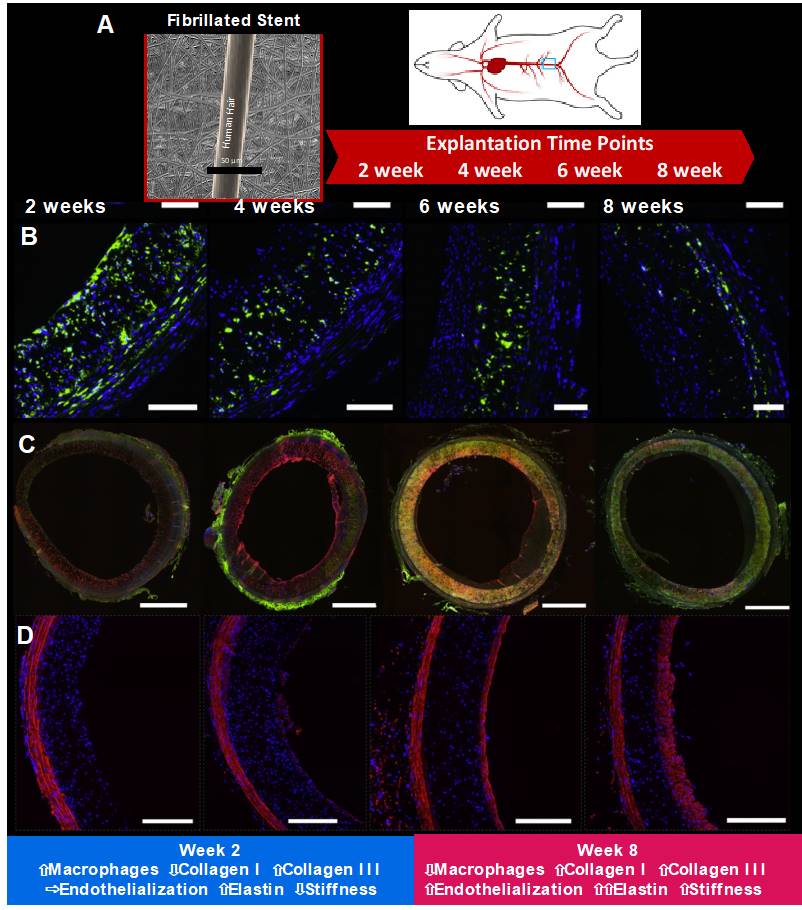
STENTiT has a unique technological platform where support grafts are composed of polymeric microfibers, processed by electrospinning. This scaffold was evaluated in the abdominal aorta of 16 rats, equally divided into four groups with follow-up times of 2, 4, 6, and 8 weeks (Panel A) (118). Panel B shows macrophage presence. Immunofluorescent co-staining of CD68 (a pan-macrophage marker) (green) and cell nuclei (blue). Panel C shows collagen types I and III. Immunofluorescent co-staining of collagen type I (green), collagen type III (red), and cell nuclei (blue). Panel D shows elastin. Immunofluorescent co-staining of elastin (red) and cell nuclei (blue).
Endovascular intervention of peripheral arterial disease (PAD) is still challenging, and compared with DES, BRSs may improve patency rates and avoid the risk of restenosis after degradation. This field has therefore gained significant interest and numerous scaffolds have been developed, with four currently being investigated in clinical trials, and two companies receiving CE-marks for their BRS for PAD.
ABSORB ESPRIT BVS system (Abbott Vascular, USA) consists of a PLLA structure coated with a 7-mm PDLLA polymer and 100μg/mm of everolimus. A series of studies have investigated the safety and efficacy of this device for PAD. Varcoe et al. reported primary patency rates of 96% and 84.6% at 12 and 24 months, respectively, and rates of freedom from ID-TLR of 96% and 96%, respectively among 38 limbs in 33 patients treated for critical limb ischemia (68.4%) or severe claudication (31.6%) . Complete wound healing occurred in 64% of those treated for tissue loss, with no major amputation and a limb-salvage rate of 100%. At 3 years, 92% of the limbs treated for tissue loss achieved complete wound healing, with no major amputations (limb salvage 100%) .
The ESPRIT I study included 35 patients with symptomatic claudication due to superficial femoral artery and external iliac artery lesions treated with the ESPRIT BVS. At 1 and 2 years, the binary restenosis rates were 12.1% and 16.1%, respectively, and TLR was performed in 3 of 34 patients (8.8%) and 4 of 32 patients (11.8%), respectively. Clinical performance was significantly improved: 71% of patients had a Rutherford–Becker class of 0 at 2 years . A multi-center international randomized trial (LIFE-BTK) evaluating the ESPRIT BVS versus percutaneous transluminal angioplasty for below knee PAD will start soon (NCT04227899).
The Igaki–Tamai BRS (Kyoto Medical Planning Co, Japan) is made of PLLA and is drug-free. The REMEDY stent is its successor for peripheral arteries with a zig-zag helical design, and a strut thickness of 0.17mm. The Igaki–Tamai scaffold received its CE mark for use in peripheral arteries in November 2007. In the GAIA study the Igaki-Tamai device was used in patients with occlusive superficial femoral artery disease with immediate angiographic results comparable to results with metal stents and high secondary patency rates (89.3% after TLR) at 1 year.
The REMEDY stent is currently being tested in two ongoing trials. The first is the ALSTER study, which is a multi-center, global study enrolling up to 600 patients, with the aim to evaluate the safety and efficacy of the REMEDY stent for the treatment of superficial femoral artery and iliac lesions. The smaller second study, named SPECTRUM, will enroll 300 patients with peripheral artery disease in Germany and Austria.
CREDENCE BtK (Meril Life Science, India) is a sirolimus-eluting BRS, with a scaffold made of PLLA, which is coated with PDLLA and 1.25μg/mm2 of sirolimus. It is characterized by a hybrid cell design, which consists of open and closed cells with a strut thickness of 100μm. Parikh presented the 12- month results of the CREDENCE Btk study which included 30 patients with critical limb ischemia and reported primary patency rates at 6 and 12 months of 93.3% and 88.9%, respectively, with no TLR or ScT by 12 months.
The MOTIV scaffold (REVA Medical, USA) is made of TyrocoreTM and coated with sirolimus at a dose of 1.97μg/mm. TyrocoreTM is composed of an iodinated, polycarbonate copolymer of the desaminotyrosine and biocompatible hydroxyesters. The struts have a thickness of 95μm and are radiopaque. The device received a CE mark for use in PAD in July 2018 , with an ongoing study (NCT03987061) now aiming to evaluate it in 15 patients with below-the-knee limb disease, with co-primary efficacy (prschemiaimary patency rate post-procedure and at 12- month) and safety (rate of serious device-related adverse events within 30 days post-procedure) endpoints.
A novel Xeltis bypass graft (XELTIS company, Netherlands) is a clinical-stage medical device developed by a company pioneering a restorative approach in cardiovascular therapy. The Xeltis bypass graft is made from a bioabsorbable supramolecular polymer. More specifically, polyester urethanes were used that contained the ureidopyrimidinone supramolecular- binding motif . Wu et al. reported one-year performance of the Xeltis bypass graft in an ovine model: early abnormal mechanical factors (bending angle, superficial wall strain and endothelial shear stress) were associated with late Quantitative Flow Ratio decrease .
The prospective, non-randomized, first-in-human clinical study (NCT04545112) started on 15 September 2020, to assess the feasibility of the Xeltis coronary artery bypass graft. The trial will include 15 patients with symptomatic coronary artery disease, with suitable multi-vessel disease and selected by the local Heart Team for elective coronary artery bypass grafts surgery of at least 3 bypass grafts (minimally 1 artery and 2 veins or 2 arteries and 1 vein). The primary outcome measures are procedural success and freedom from device-related serious adverse events during the first 30 days. The secondary outcomes are intimal hyperplasia area, graft patency, lumen diameter uniformity, freedom from major adverse cardiac and cerebrovascular events, freedom from vein harvesting related wound infection, non-infective wound healing disturbances and leg pain, and a composite of all causes of mortality. The safety and efficacy evaluation of this Xeltis bypass graft implantation in human is still ongoing.
New generation DES represent the latest revolution in percutaneous intervention and are considered the best currently available technology in the field, but there remains room for improvement, especially regarding very long-term clinical outcomes with these late events presumably stemming from the continued presence of a metallic cage.
BRS have been developed to provide drug delivery and mechanical support functions similar to metallic drug-eluting stents, followed by complete resorption with recovery of more normal vascular structure (e.g. expansive vessel wall remodeling, , Figure 17) and function (, Figure 18), potentially improving very late clinical outcomes.
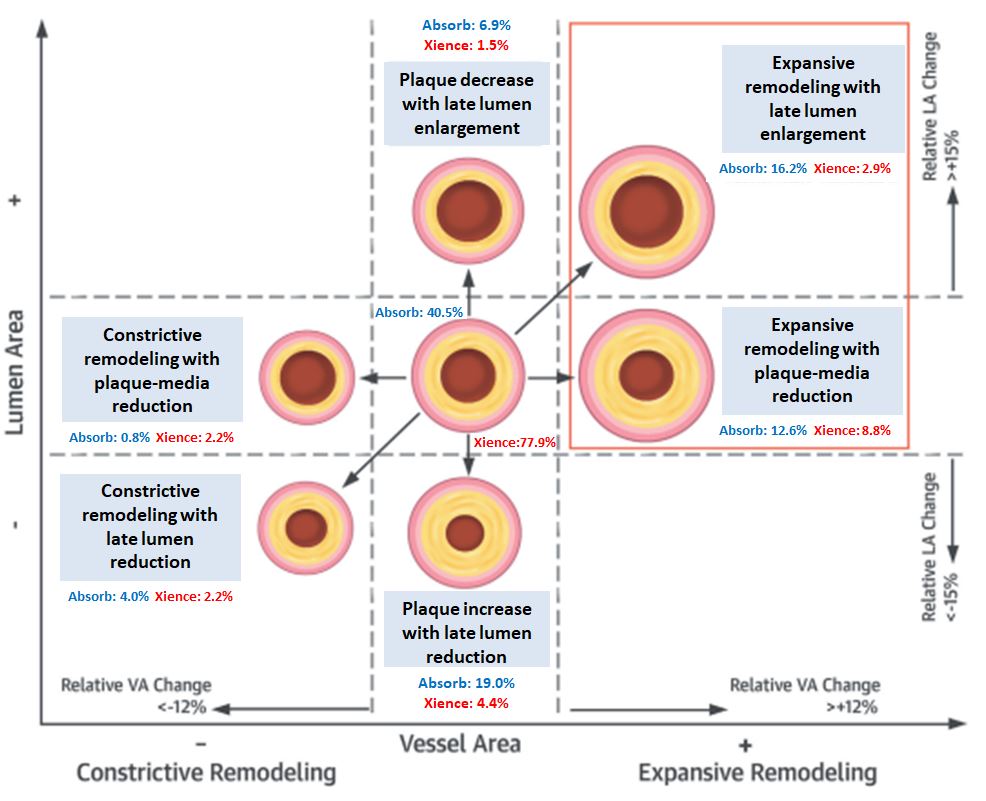
Relative changes in vessel area (VA) (constrictive remodeling <12%, expansive remodeling > +12%) and relative changes in lumen area (LA) (lumen reduction <15%, lumen enlargement > +15%) defined remodeling patterns. No lesions showed constrictive remodeling with LA increase or expansive remodeling with LA decrease. Expansive vessel wall remodeling was more frequent with the bioresorbable vascular scaffold (BVS) than the metallic drug-eluting stent (DES) and could be determined by patient baseline characteristics and periprocedural factors (128).
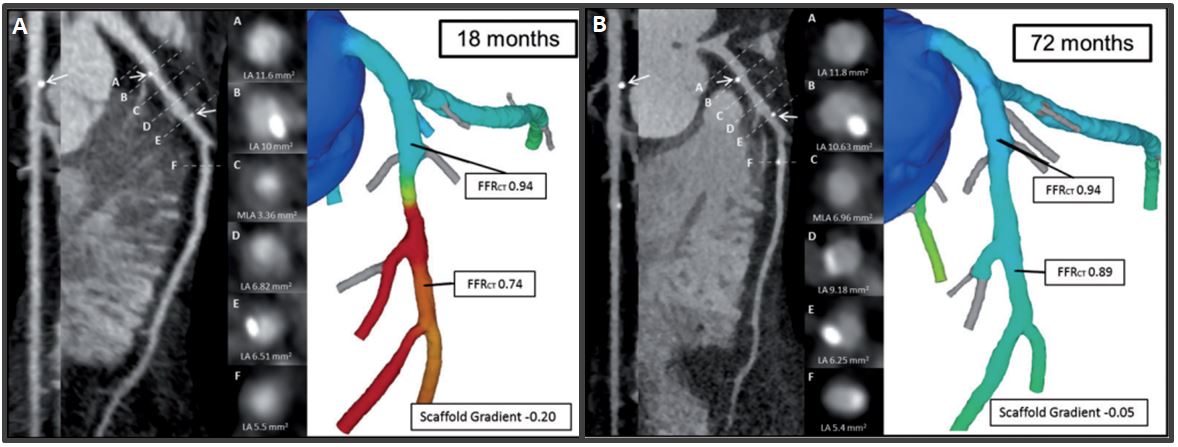
Case example of serial MSCT with FFRCT at follow-up. Case example of a patient treated with a Bioresorbable Vascular Scaffold (white arrows) in the proximal segment of the left Circumflex artery. At 18 months (A), MSCT Angiography showed a patent scaffold with MLA of 3.1 mm2 and FFRCT scaffold gradient of 4. At 72 months (B), the persistence of the normalization of the FFRCT in-scaffold with the same gradient was found. However, in the distal edge of the scaffolded region plaque progression with a reduction of the luminal area and FFRCT was observed (130).
After the encouraging results of ABSORB B , several large registries and RCTs comparing the Absorb BRS to the Xience were conducted , , . However, the Absorb suffered a major setback when the results of the co-primary endpoints did not meet the hypothesis. Namely, quantitative differences in vasomotion were not observed between the devices, late loss in the Absorb BRS was significantly larger than in the XIENCE stent, and mid-term results showed a higher risk of both ScT (, Figure 19) and TLF for the Absorb BRS, the latter driven mainly by increased rates of TV-MI and ID-TLR (, , , , , , ).
To date, sufficient mid-term clinical data are now available to support the fact that Absorb BRS is inferior to new generation DES , and both the FDA and the ESC-EAPCI have issued documents reflecting this.
Recent meta-analysis of four ABSORB BRS trials (ABSORB II, III, Japan, and China) demonstrated that although rates of adverse ischemic events cumulative through 5 years were increased when compared with the EES group, the period of excess risk ended at 3 years . Hence if new-generation BRSs can demonstrate comparable results with metallic DES during their active biosorption phase, they may prove to be acceptable alternatives to metallic DESs. The ultimate judge of the Absorb BRS could probably be the on-going ABSORB IV trial, that has reported 30-day and 1-year results .
For the BRS technology overall, at present hope seems to lie more on the development of second-generation devices and approximately 34 BRSs from 22 companies are currently under development. The potential device-specific factors related to the increased event rate in Absorb were: 1) weak mechanical properties, 2) larger strut thickness (less embedment and larger protrusion) and width (larger footprint) predisposing to underexpansion/protrusion of struts, eventually resulting in increased thrombogenicity, and 3) longer bioresorption time combined with failure of encapsulation of struts before the dismantling process ensues.
Given the diversity of mechanical properties, absorption process, and drugs eluted of each new BRS, one could expect considerable difference in early and late clinical outcomes. As a matter of fact, several second-generation devices have shown encouraging results in clinical trials such as BIO-MAG-I trial, and they continue to challenge a class effect of their prior unfavourable results .
The new-generation devices should be investigated in RCTs against a mature technology with solid results, like DES , . In spite of the present unfavourable clinical results, a number of companies worldwide, especially in Asia, have initiated ambitious RCTs for their products, that will determine if BRS evolutional history will follow the pattern of DES, in terms of success through further development, or another scenario will prevail.
Francesco Prati, Flavio Giuseppe Biccirè
Updated on May 13, 2021
Soo-Teik Lim, Tian-Hai Koh
Updated on February 1, 2018
Scot Garg, Sharmaine Thirunavukarasu, Raffaele Piccolo, Patrick W. Serruys, Stephan Windecker
Updated on January 23, 2018
Bruno Scheller
Updated on May 13, 2021