Francesco Prati, Flavio Giuseppe Biccirè
Updated on May 13, 2021
Drug coated balloons (DCB) represent a clinical treatment modality for non-stent based local drug delivery in coronary and peripheral artery disease. Their advantages over standard angioplasty and stent technologies include a homogeneous drug delivery to the vessel wall, an immediate drug release without the use of a polymer, the option of using balloon catheters alone or in combination with a stent, no foreign object left behind in the body, the potential of reducing antiplatelet therapy, and a lower restenosis rate in some indications. As with drug eluting stents (DES), one cannot assume a class effect for DCB. Data from randomised clinical trials identify the treatment of coronary in-stent restenosis (ISR; ESC recommendation 1A), of coronary de novo lesions in risk indications (e.g. small vessels and high bleeding risk), and of de novo and restenotic lesions in peripheral artery disease (new standard of care in the SFA) as viable options. Furthermore, treatment of de novo lesions in bifurcation lesions, long lesions, paediatric interventions, and cerebrovascular applications are potential beneficial indications. In the coronary application, a strategy of DCB-only (lesion preparation followed by DCB angioplasty alone or stenting in case of severe dissection) may become a better alternative in small vessel disease, long and complex lesions, or bifurcations.
Coronary angioplasty was introduced clinically by Andreas Grüntzig in 1977 . Later on, coronary stents were developed to cover flow-limiting dissections to prevent early vessel closure . It was shown that bare metal stents (BMS) lead to a significant reduction of restenosis compared to conventional angioplasty (POBA) . As a consequence, primary BMS implantation became the preferred treatment strategy in coronary interventions, ignoring the knowledge that a provisional stenting rate of 20 to 40% would have been sufficient .
A common misunderstanding of today’s interventional cardiologists is their belief that ‘stents let them sleep at night’. In the early days of stent implantation acute stent thrombosis rate was in the double digits. The problem disappeared with the introduction of dual antiplatelet therapy in the second half of the 1990s (this innovation in reality allowed ‘sleep at night’ after a stent procedure). Later on, results were further improved by high-pressure dilatation and thinner struts.
In the beginning of the new century, local intravascular drug delivery by drug eluting stents (DES) seemed to cross the last frontier in interventional vascular procedures, namely restenosis by inhibition of neointimal proliferation induced by the permanent implant. However, the need for long-term dual antiplatelet therapy limits the outcomes of this technology. Sustained drug release seems to be essential for stent-based local drug delivery due to the inhomogeneous drug distribution from a drug-eluting stent to the arterial wall . About 85% of the stented vessel wall area is not covered by the stent struts resulting in low tissue levels of the antiproliferative agent in these areas . Therefore, high drug concentrations on the stent struts, including a controlled and sustained release, are mandatory for stent-based local drug delivery with the consequence of delayed and incomplete endothelialisation of the stent struts . Furthermore, polymeric matrixes on the stent embedding the antiproliferative drug could induce inflammation and thrombosis. Neoatherosclerosis induced by the permanent drug eluting implant is becoming a long-term issue of DES .
Important to note that this unlimited enthusiasm for stenting remains focused on coronary interventions. In peripheral artery disease, stent use remains limited due to external forces on the vessel resulting in stent fractures, unfavorable anatomies, and long diffuse disease .
New concepts to overcome the limitations of metallic stents should promote endothelialisation, avoid permanent implants, and lead finally to the reestablishment of normal vascular function (“vascular restoration”) (Table 1). Fully bioabsorbable vascular scaffolds (BVS) meet these requirements. However, concerns have been raised that such current generation devices may include an increased risk for stent thrombosis and myocardial infarction .
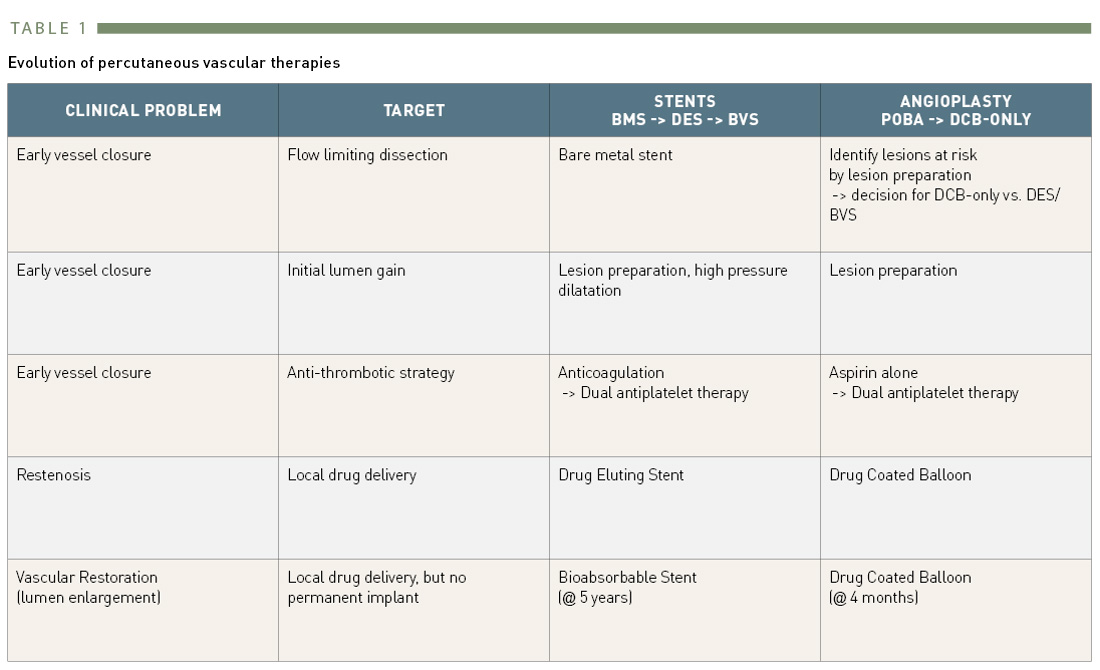
Evolution of percutaneous vascular therapies
To the best of our knowledge, drug coated balloons for restenosis inhibition were first mentioned in the literature in 2004 . Antiproliferative taxane compounds, such as paclitaxel, seemed to be suitable for non-stent based local intravascular restenosis prevention due to their high lipophilicity and tight binding to various cell constituents, resulting in effective local retention at the site of delivery. The addition of an excipient, e.g. a contrast agent surprisingly resulted in a solubility of taxanes far beyond the concentrations applied in previous investigations. In the porcine coronary model, the intracoronary bolus administration of a taxane contrast medium formulation led to a significant reduction of neointimal formation after experimental coronary stent implantation despite the short application time . Paclitaxel in the contrast agent was better tolerated and led to higher local tissue concentrations than diluted TaxolTM, indicating the impact of additional compounds for local drug transfer . The surprising discovery was that sustained drug release is not a precondition for long lasting restenosis inhibition.
In 2001, the basic premise of a more lesion than vessel specific method of intramural drug delivery became embodied in a DCB concept (Figure 1) . By coating paclitaxel onto the surface of a conventional angioplasty balloon used to dilate the stenotic artery, an exclusively local effect could theoretically be achieved, with the drug transferred to the dilated segment as the balloon is inflated. In this way, an effective local drug concentration is achieved with very low systemic exposure. However, there are several properties of DCB that are crucial for ensuring effective drug delivery to the target site, including: (a) the form of balloon surface, (b) the homogeneity of distribution along the surface of the balloon, (c) drug stability during production, handling and storage, (d) the degree of premature loss while transiting to the target vessel segment, (e) the ability to release during balloon expansion, (f) the transfer efficiency to the vessel wall, (g) the amount of particulate material released to the distal circulation .
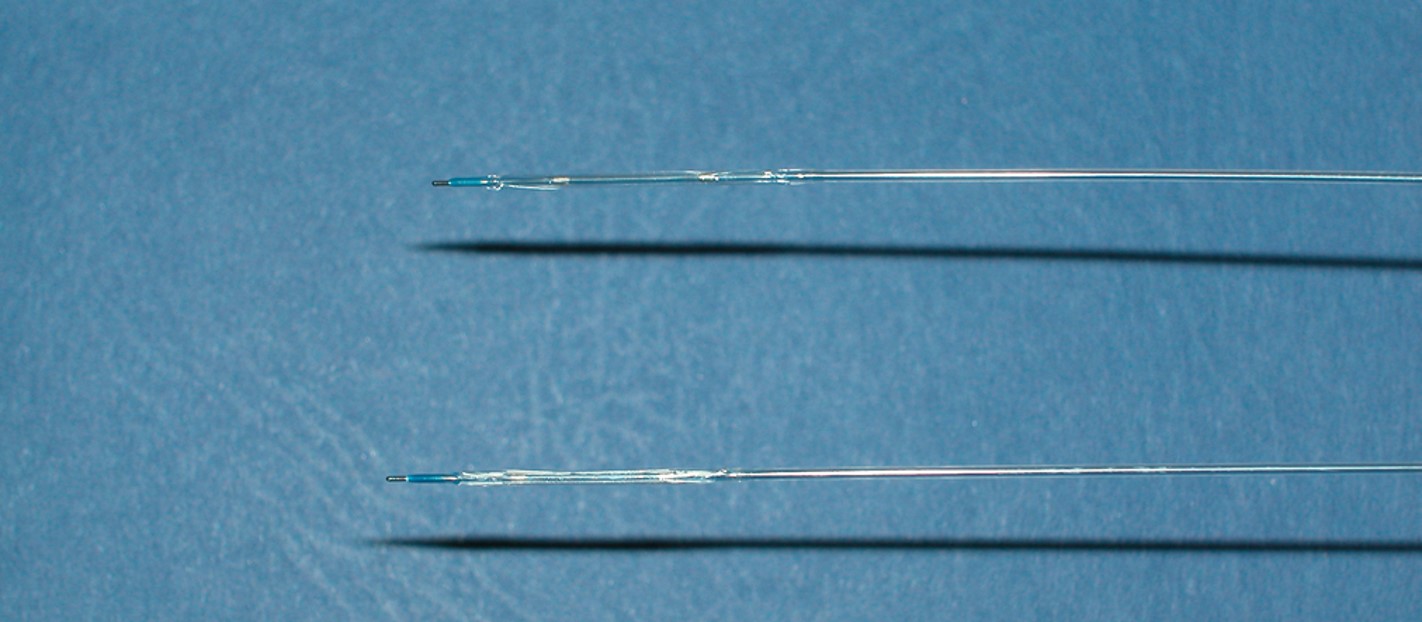
Example of a drug coated balloon alongside a standard angioplasty balloon The drug coated balloon has an opaque surface which distinguishes it.
The basic in vitro and in vivo experiments leading to the concept of drug coated balloons identified a specific matrix coating with paclitaxel in combination with a small amount of the hydrophilic x-ray contrast medium iopromide (Ultravist™; Bayer Schering Pharma AG, Berlin, Germany) to be effective (Paccocath™) (Figure 1) . The balloons were angioplasty balloons coated with a paclitaxel dose between 1.3 and 3 μg/mm2 of balloon surface. About 10% of the initial amount of paclitaxel on the balloon was lost while the catheter was advanced towards the lesion through the haemostatic valve and the guiding catheter, and about 80% of the dose was released during inflation. Most of the dose released at the target site is distributed as particulate matter distally in the blood stream with less than 20 % being directly taken up into the vessel wall. At five week follow-up, the implantation of stents pre-mounted on paclitaxel coated balloons was found to have caused a marked, dose-dependent, and statistically significant reduction in angiographic late lumen loss (LLL) and an equally impressive, statistically significant increase in minimal lumen diameter compared with controls. Quantitative coronary angiography revealed no edge effects or signs of malapposition or aneurysm. Histomorphometry showed a statistically significant increase in lumen diameter and lumen area and a corresponding decrease in maximal neointimal thickness and neointimal area in the vessels treated with paclitaxel coated balloons (reduction of neointimal area by 63% in the paclitaxel coated balloon group versus the uncoated balloon group) (Figure 2 .) Furthermore, the drug was more evenly distributed on the vessel surface compared to a drug-eluting stent .
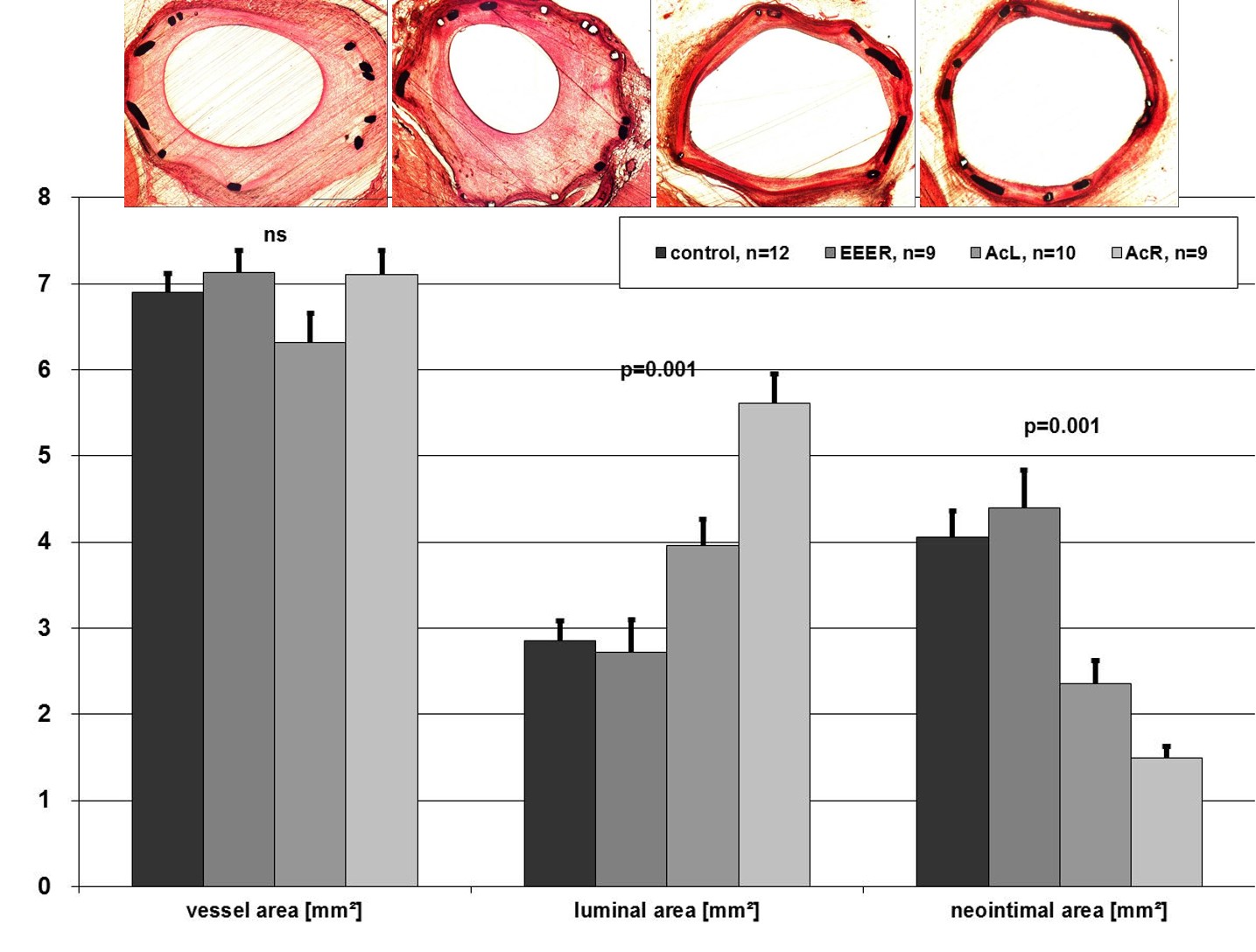
Histology and histomorphometry of stented, porcine coronary arteries after 35 days.
Implantation of bare-metal stents with conventional PTCA catheters (control) and paclitaxel-coated, PTCA catheters with EEER coating or Ac coating (AcL and AcR).
Parameters shown are injury score, vessel area, luminal area, neointimal area, and maximal neointimal thickness. Abbreviations are as defined in text. Values are mean±SD, n=40.
EEER=ethyl acetate as the solvent with ≈2 μg paclitaxel/mm2 balloon surface,
AcL=acetone as solvent with1.3 μg paclitaxel/mm2 balloon surface (low dose paclitaxel)
AcR=acetone as solvent with 2.5 μg paclitaxel/mm2 balloon surface (regular dose)
Since this initial research was published several manufacturers have started developing or commercializing DCB. Currently, paclitaxel is the drug of choice with the typical dosage being 2-3.5 μg/mm² balloon surface. In Europe, regulatory approval exists for different coronary and peripheral devices. Furthermore, three peripheral devices gained FDA approval since the end of 2014 (Table 2). The critical factors enabling successful drug transfer are the formulation used to coat the balloon and the coating process. Current products range from those with no additive and very tight binding of the drug to the balloon membrane to those applied in conjunction with standard contrast agents or other beneficial additives.
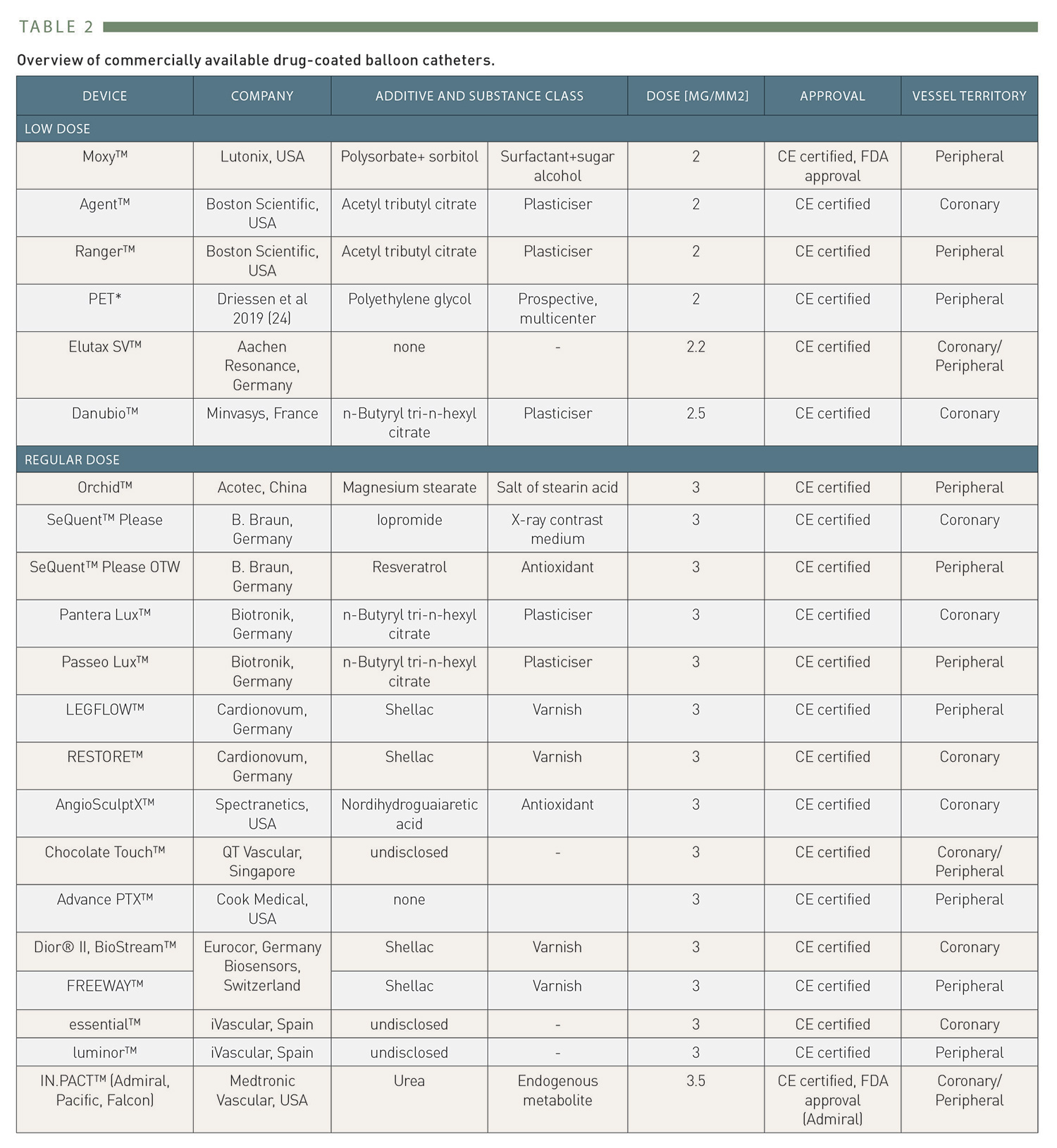
Overview of commercially available drug-coated balloon catheters.
Paclitaxel concentrations in the vessel wall immediately after DCB inflation are far above its solubility representing the sum of dissolved (i.e. the pharmacologically active drug) plus solid crystalline paclitaxel which serves as store but does not display toxicity or pharmacological effects . The low solubility prevents immediate dissolution following contact with blood, limits loss of paclitaxel from balloon, modulates efficacy and toxicity by limiting the maximum achievable concentration, and contributes to the long lasting efficacy . Biologically effective cytotoxic tissue levels can therefore not even be reached within the first 24 hours . The local dose administered from the DCB is about 750 times lower in the case of coronary paclitaxel-DCB compared to systematic cancer therapy, which excludes systemic effects of this local therapy.
The risk of forming emboli if particles of the coating are released in the blood has been discussed extensively . However, this theoretical problem of soluble paclitaxel particles has been largely overestimated. In contrast, the release of embolic debris with lower solubility and in a far larger mass and volume as an unavoidable consequence of angioplasty and stent implantation has been neglected widely .
In-Stent Restenosis (ISR)
Compared to BMS-ISR, DES-ISR represents a high-risk scenario since stent-based local drug delivery failed in the first attempt . Therefore, outcomes after treatment of DES-ISR are inferior to treatment of BMS-ISR, independent from the selected approach. A network meta-analysis identified the implantation of an everolimus eluting stent (EES) or the use of a drug coated balloon (DCB) as the two most effective measures to reduce recurrence within the first year . The 2014 and 2018 ESC guidelines for revascularization give the same highest level of recommendation for the stent-in-stent approach and the use of DCB in the treatment of BMS- and DES-ISR (class 1 level A, each) .
The PACCOCATH In-Stent Restenosis (ISR)-I trial was a German, controlled, randomised, first-in human, multicentre study with blinded angiographic evaluation which compared the efficacy and tolerance of Paccocath™ paclitaxel coated balloon catheters with conventional uncoated catheters for the treatment of coronary in-stent restenosis. Compared to patients treated with an uncoated balloon, patients in the Paccocath™ balloon group had significantly better angiographic results (in-segment late luminal loss 0.74±0.86 mm versus 0.03±0.48; p=0.002) and concomitant 12-month clinical outcomes . The subsequent Paccocath ISR-II trial extends the initial findings from the first ISR trial. The second trial reproduced the ISR-I results in an additional 56 patients with similar baseline clinical and angiographic data. The most surprising finding was that the beneficial effects of the Paccocath™ balloon catheter were maintained for up to 5 years after the intervention . Importantly, in contrast to DES, combined antiplatelet therapy was continued only for one month followed by treatment with aspirin alone.
The SeQuent™ Please DCB (B.Braun Melsungen AG, Germany) using the paclitaxel iopromide coating (PaccocathTM) has been clinically studied in the PEPCAD (Paclitaxel-eluting PTCA-Catheter in Coronary Artery Disease) clinical trial program confirming the results of the FIM Paccocath trial, comparing DCB with POBA and sandwich DES, and expanding it to DES-ISR. The trials with SeQuent™ Please , , , , , , , , , , lead to the class I level A recommendation for the treatment of BMS- and DES ISR by DCB in the current ESC guidelines . A comparison of two different devices (In.Pact FalconTM , Medtronic vs. Dior IITM, Eurocor) supports the assumption that there is no class effect (e.g. LLL -0.03 ± 0.43 vs. 0.36 ± 0.48 mm, p = 0.014 in favor of In.Pact Falcon DCB) .
The key element to improve angiographic outcomes is careful lesion preparation to assure sufficient initial lumen gain. Tools to reach this goal are predilatation with semi- and non-compliant balloons, and specialty balloons like scoring balloons. The ISAR-DESIRE 4 trial showed that in patients with DES-ISR, predilation with a scoring balloon before use of a DCB was associated with a significant reduction of diameter stenosis and binary restenosis at follow-up angiography as compared to ‘simple’ predilatation with a semi-compliant balloon . Another option could be a drug coated scoring balloon to combine improved lesion preparation and local drug delivery .
An interesting finding from the ISAR DESIRE III as well as RIBS IV and V trials is the different handling of therapy failure after DES or DCB for ISR treatment. The willingness for reintervention appears to be significantly lower in the presence of several stent layers than after DCB angioplasty. This automatically leads to an overestimation of the clinical endpoint of revascularization, which at least partly explains the significant difference in the major adverse cardiac events in RIBS IV after one year. On the other hand, this means that DCB leaves more therapeutic options open for ISR treatment.
The DAEDALUS patient level meta-analysis reported a slightly higher target lesion revascularization rate (TLR) after DCB with concomitant reduction of hard clinical endpoints such as death and myocardial infarction by the use of paclitaxel coated balloons as compared to DES in ISR treatment . Larger randomized studies (ISAR DESIRE III, PEPCAD DES, PEPCAD China) report a survival benefit for DCB in DES-ISR treatment at longer follow-up (2 to 3 years) , , . For example, after 3 years in ISAR DESIRE 3, hazard ratio for overall mortality was 0.38 (6.0 vs. 15.3 % p=0.02) and 0.27 for cardiac mortality (p=0.03) in favor of DCB vs. DES. Important to note, this mortality benefit was not related to re-intervention rates . This finding may be explained by an elevated stent thrombosis risk with sandwich DES .
DCB treatment as default strategy for DES-ISR avoids several layers of metal, reduces the need for prolonged dual antiplatelet therapy, allows for repeatability of the procedure, and apparently positively influences hard endpoints on long-term. The disadvantage in angiographic outcomes could be limited by careful lesion preparation assuring sufficient initial lumen gain including specialty balloons (Figure 3).
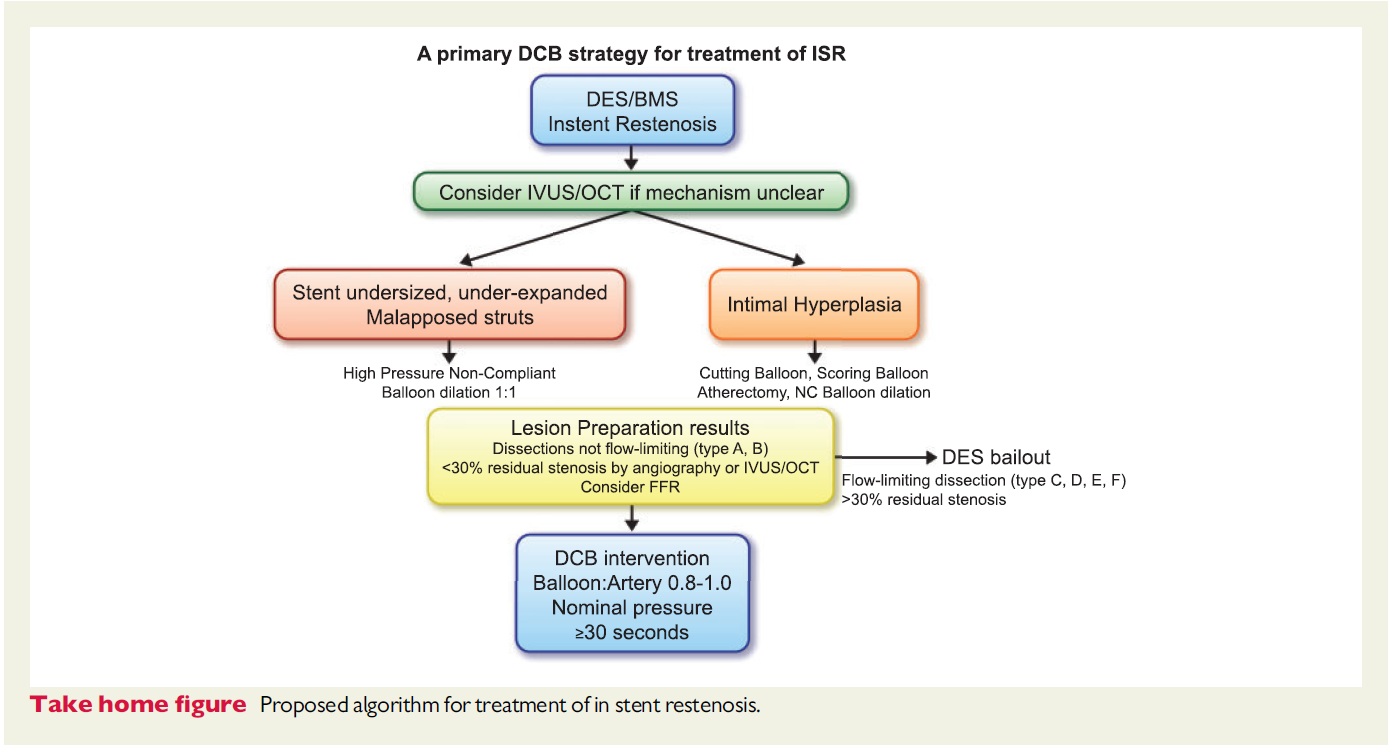
From Lansky et al., Eur Heart J. 2020 Oct 7;41(38):3729-3731
Coronary de-novo disease
To date, DCB therapy has only been recommended for the treatment of coronary in-stent restenosis . This indication is widely accepted since the use of a paclitaxel-coated balloon avoids an additional stent layer and may reduce the probability of death or myocardial infarction in the long term . Initial attempts of combining DCB with BMS to create a new type of ‘polymer free DES’ were inferior to limus-DES . Patients undergoing standalone DCB treatment in coronary small vessel disease (SVD) showed superior long-term outcomes compared to the combination of DCB and BMS in the same segment . As a consequence, an expert consensus group proposed the so-called “DCB-only” strategy. The general principle underlying the recommendations includes a careful lesion preparation with a balloon to vessel ratio of 0.8-1.0. Depending on the result after lesion preparation the operator can decide whether to proceed with a DCB stand-alone strategy in case of an acceptable result or to use a stent (or maybe in the future a next generation scaffold) in case of major dissection (type C or higher), significant residual stenosis or reduced flow (Figure 4) .
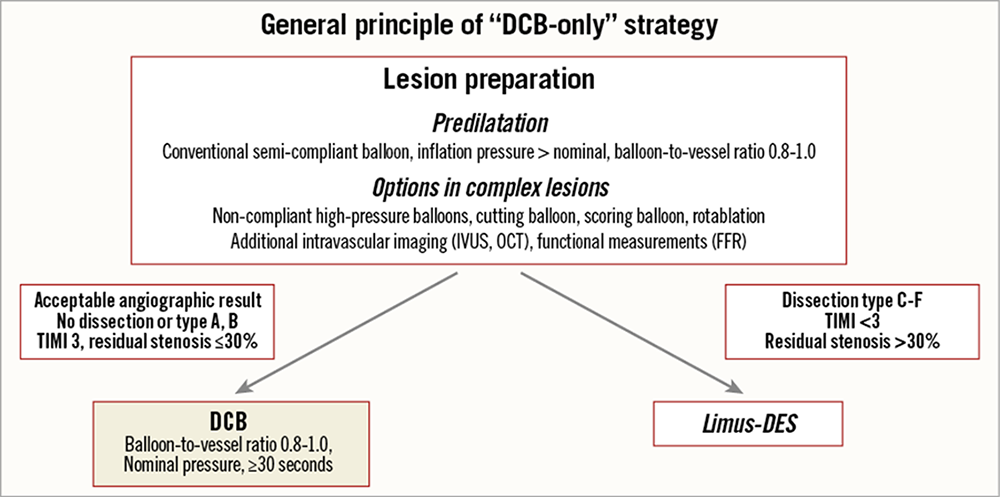
Consensus group algorithm for the use of drug coated balloon in practice
For the majority of interventional cardiologists, however, it is still difficult to imagine treating a lesion primarily without a stent. The argument against DCB is usually based on the cosmetic acute result and an allegedly high number of acute vascular occlusions. MACE rates in large registries at 9 months were 4.7 % in SVD and 2.6 % in larger coronary arteries . Important to note, there was no safety signal in terms of early vessel closure. A propensity matched cohort from the SCAAR registry including 1,197 DCB and 1,197 current generation DES showed a significant lower rate of target lesion thrombosis after ‘DCBonly’ with a hazard ratio of 0.18 (0.2 vs. 1.1%) . In the BASKET SMALL 2 and DEBUT trial no acute vessel closure in only DCB treated lesions occurred , . This may be explained by the fact that lesions at risk are identified by lesion preparation and undergo stenting. Another factor is dual antiplatelet therapy (DAPT) which was never investigated in angioplasty alone; however, there is no reason to deny a similar beneficial impact of DAPT in DCBonly as it was the case of stenting . Lesions with non-flow limiting dissections undergoing angioplasty show excellent clinical outcomes . In peripheral artery disease, DCB efficacy was increased in more complex dissections . In the rare event of a flow-limiting dissection after successful lesion preparation and DCBonly, a limus eluting stent should be used for bailout .
The BELLO (Balloon Elution and Late Loss Optimisation) trial was a randomised, multicentre study with 182 patients to assess LLL at six months in coronary SVD after treatment with either In.Pact™ Falcon DCB (Medtronic, Ireland) or Taxus™ stent. 97% of the patients in the DCB-arm and 81% receiving a DES underwent lesion preparation. LLL after 6 months was significantly lower in the DCB arm. After 3 years a significant difference in MACE in favor of the DCB was reported which was primarily driven by death and myocardial infarction . Findings from SVD in the randomized PEPCAD Japan study support the importance of lesion preparation. Patients undergoing predilatation with a scoring balloon (NSE Alpha) showed a significant higher MLD post intervention and a significant reduction of the need for bailout stenting or a residual stenosis of more than 50% after angioplasty . In the BASKET-SMALL 2 trial 758 patients with de novo lesions (<3 mm in diameter) were randomized to receive angioplasty with SeQuent™ Please DCB versus implantation of a second-generation DES, predominantly everolimus eluting stents. Non-inferiority of DCB versus DES was shown. After 12 months, MACE were similar in both groups (7·5% for the DCB group vs 7·3% for the DES group; p=0·9180) . After 3 years, DES and DCB continued to show equal event rates .
The DEBUT trial compared SeQuent™ Please with a high bleeding risk population. At 9 months, the primary end point consisting of cardiovascular mortality, non-fatal myocardial infarction or ischemia-driven TLR occurred in 1 patient in the DCB group and in 15 patients in the BMS group with a risk ratio of 0.07. This benefit of DCB therapy over BMS was maintained up to three years, mainly driven by improved survival in the paclitaxel coated balloon group .
PEPCAD NSTEMI compared the clinical outcomes of patients with Non-ST-Elevation Myocardial Infarction (NSTEMI) treated with either SeQuent™ Please or stent. Two hundred and ten patients with NSTEMI were enrolled in a randomized, controlled, non-inferiority multicenter trial. In the stent group, 56% of patients were treated with BMS, 44% with current generation DES. In the DCB group, 85% of patients were treated with DCB only whereas 15% underwent additional stent implantation. During a follow-up of 9.2 ± 0.7 months, DCB treatment was noninferior to stent treatment with a TLF of 3.8% vs 6.6% (intention-to-treat, p=0.53). There was no significant difference between BMS and current generation DES. Total MACE rate was 6.7% for DCB vs 14.2% for stent treatment (p=0.11), and 5.9% vs. 14.4% in the per protocol analysis (p=0.056), respectively .
The role of DCB in bifurcations may be a puristic approach with DCBonly in main and side branch or the use of limus-DES in the main branch and DCBonly in the side branch. Two RCT showed a significant reduction of LLL and binary restenosis in the sidebranch by the use of DCB (primary endpoint LLL in PEPCAD-BIF 0.13 mm in the DCB vs 0.51 mm in the POBA group, p = 0.013) , . Further interesting indications are chronic occlusions (CTO) to reduce metal payload and patients with acute ST-elevation myocardial infarction.
Lumen enlargement during angiographic follow-up after POBA is a rare event . In a series of 58 consecutive patients with native coronary artery lesions treated with DCBonly, target lesion minimal lumen diameter increased significantly within 4.1 ± 2.1 months (1.75 ± 0.55 vs. 1.91 ± 0.55 mm, p < 0.001, diameter stenosis 33.8 ± 12.3 vs. 26.9 ± 13.8 %, p < 0.001), while there were no changes in non-target reference vessel diameters (2.33 ± 0.60 vs. 2.34 ± 0.61 mm, p = ns). A total of 69 % of patients showed luminal enlargement whereas 29 % had minor luminal loss . In a prospective study in 27 patients, 28 de novo lesions were successfully treated with DCB only. Angiographic late luminal loss was 0.02 ± 0.27mm. Mean vessel and lumen areas (IVUS-VH) showed a significant increase at 9 months (12.0 ± 3.5 mm2 to 13.2 ± 3.9 mm2, p <0.001; and 5.4 ± 1.2 mm2 to 6.5 ± 1.8 mm2, p <0.001, respectively). Although mean plaque area was unchanged (6.6 ± 2.6 mm2 to 6.6 ± 2.4 mm2, p = 0.269), percent atheroma volume decreased significantly (53.4 ± 7.9 % to 49.5 ± 6.4 %, p = 0.002) . Recent data additionally provide evidence for an influence on plaque burden itself .
Current consensus of experienced DCB operators is that the best indication for DCB in coronary arteries is de novo disease since all the requirements for the concept of leaving nothing behind are fulfilled. A recent meta-analysis including 4,590 patients shows a reduced risk of myocardial infarction in the first year and possibly a reduction of mainly cardiac deaths after 3 or more years for the use of DCB coronary .
Femoro-popliteal artery
It was unclear whether the positive findings from the coronary Paccocath ISR-I and II studies could be transferred to restenosis prevention in the peripheral arteries. Thus, shortly after initiation of the coronary ISR trial, two additional trials included patients with de novo stenosis and occlusion as well as restenosis in the superficial femoral or popliteal arteries , . Both Thunder and FemPac trials were German, randomised, multicentre studies with evaluation of the primary endpoint by a blinded core lab. They compared Paccocath™ paclitaxel coated and conventional uncoated balloon catheters with regard to efficacy and tolerance in inhibiting restenosis in the superficial femoral artery. In the Thunder trial, a total of 154 patients with stenosis or occlusions of the superficial femoral or popliteal arteries were enrolled including a third treatment arm with paclitaxel dissolved in the contrast medium, i.e., Ultravist™. At six month follow-up, treatment of patients with Paccocath™ balloons was found to be associated with significant reductions in LLL compared to patients of the uncoated balloon group or patients treated with paclitaxel dissolved in the contrast medium. Importantly, the rate of target lesion revascularisation at six, twelve, and twenty four months post-intervention remained significantly lower in the Paccocath group compared with both other groups . In the FemPac trial, 87 patients were randomly assigned to the treatment with either standard balloon angioplasty or the Paccocath™ balloon. At six month follow-up, patients treated with the Paccocath™ balloon had significantly reduced LLL compared to the control group. The number of target lesion revascularisations was significantly lower in the Paccocath group than in control subjects, and the difference between both treatment groups was maintained eighteen to twenty four months post-intervention. Furthermore, patients in the coated balloon group also had improvement in Rutherford class, but no difference in the improvement in ankle brachial index was found. Compared to the Thunder trial, LLL in the control group was lower in this study, and therefore, the difference to the Paccocath group was smaller. Nevertheless, the FemPac trial confirmed the results of the Thunder trial, demonstrating that short-term exposure of injured peripheral arteries to paclitaxel may be sufficient to inhibit restenosis . Meanwhile, 5 year data from Thunder show a persistent benefit of the DCB over conventional PTA without any signs of late catch-up. However, only patients who had not previously been revascularized and were available for follow-up were included in this long-term analysis; as a result, DCB patients were over-represented . Unfortunately, a higher total number of deaths in this subgroup was misused for a methodologically poor meta-analysis as alleged evidence of a higher mortality rate after DCB in the long-term .
In peripheral artery disease coatings different from the original Paccocath generated a wider dataset. Several first-in-man randomized trials in femoro-popliteal lesions have shown favorable outcomes in terms of LLL, binary restenosis rate, and freedom from target lesion revascularization (TLR) when comparing conventional balloon with DCB .
The FreePac™ coating (In.Pact™ DCB family; Medtronic, Ireland) is a hydrophilic coating formulation with urea as matrix substance. Urea is a nontoxic, ubiquitous endogenous compound, commonly used in pharmacy and meant to enhance the release of paclitaxel during the short time of contact with the vessel wall. In the porcine coronary model, similar amounts of paclitaxel were transferred to the vessel wall with the Paccocath™ coating and the FreePac™ coating including a very similar biologic response . In the PACIFIER trial, patients with symptomatic femoro-popliteal atherosclerotic disease were randomised to In.Pact™ Pacific or uncoated balloons (n=91). Average lesion length was 7.0 ± 5.3 and 6.6 ± 5.5 cm for DCB and control arm, respectively. At six months, LLL was -0.01 mm for DCB versus 0.65 mm for PTA (p=0.001), fewer binary restenoses (9% versus 32%), and fewer major adverse events (7% versus 35%) . Clinical follow-up up to 5 years showed a numerical survival benefit in DCB treated patients (mortality 14% vs 30% after uncoated PTA). In the IN.PACT SFA trial 331 patients with claudication or rest pain due to superficial femoral lesions were enrolled (150 subjects at 13 European centers and 181 subjects at 44 US sites). All relevant outcomes were evaluated by blinded core labs. After one year, the DCB group was superior to PTA in terms of primary patency (82% vs. 54%), clinically driven TLR (2.4% vs. 21%), primary sustained clinical improvement (upgrade in Rutherford classification ≥ 1 class in amputation- and TVR-free surviving patients; 85% vs. 69%), primary safety endpoint (freedom from 30-day device- and procedure-related death and from target limb major amputation and clinically driven TVR through 12 months; 96% vs. 77%), and MACE (death, clinically driven TVR, target limb major amputation, and thrombosis; 6% vs. 24%)]. At 24 months, patients treated with the In.Pact DCB showed significantly higher primary patency when compared with PTA (78.9% vs. 50.1%; p < 0.001). The rates of CD-TLR were 9.1% and 28.3% (p < 0.001) for the DCB and PTA groups, respectively. However, there was a difference in overall mortality after 2 years with the lowest mortality ever reported in a PTA group, while mortality in the DCB group was in the expected range . Through 5 years, patients treated with the IN.PACT Admiral DCB demonstrated a sustained treatment effect with superior freedom from clinically driven target lesion revascularization when compared with PTA. The primary safety composite was achieved in 70.7% of subjects in the DCB and 59.6% in the PTA groups (P=0.068). There were no device- or procedure-related deaths in either group . An independent patient-level meta-analysis of 1,980 patients including patients from the IN.PACT SFA trials in US, EU and Japan as well as the IN.PACT Global Clinical Study revealed no significant difference in all-cause mortality between DCB and PTA through 5 years. Within DCB patients, there was no correlation between level of paclitaxel exposure and mortality .
The Moxy™ balloon (Bard Lutonix, USA) is coated with 2 μg/mm² paclitaxel using polysorbate and sorbitol as excipients . Initial experimental data for a 3 μg/mm² formulation of this coating in the porcine coronary model indicated a similar effect to iopromide paclitaxel coated balloon catheters, comparative data for the lower concentration are not available. The LEVANT I trial was a German/Belgian, randomized, two-arm study comparing the Moxy™ DCB vs. standard balloon angioplasty for the treatment of stenosis in the femoro-popliteal arteries in 101 patients. The primary endpoint LLL at six months was significantly reduced (0.46 mm vs. 1.09 mm) by Moxy™. TLR rate did not differ significantly between the two treatments (13% vs. 22%) . In the LEVANT II pivotal trial including 476 pat(73)ients, primary patency at 12 months defined as freedom from both restenosis and TLR was 65.2% for the DCB and 52.6% for control angioplasty (p=0.015). However, freedom from clinically driven TLR was similar in both groups (38.0 vs. 37.5%) .
The ILLUMENATE study investigated a polyethylene glycol paclitaxel coated balloon (Stellarex™; Phillips, The Netherlands). 294 patients were randomized (3:1) to treatment with a DCB or an uncoated PTA. Primary patency at 2 years was significantly higher in the DCB cohort. The rates of all-cause and cardiovascular-related mortality were similar between groups.
The AcoArt I trial randomized 200 patients to a magnesium stearate paclitaxel coated balloon (OrchidTM; Acotec Scientific, Beijing, China) or uncoated PTA. Patients were in Rutherford stages 2 through 5, with a mean lesion length of 150 mm. Mean late lumen loss was 0.05 mm with coated balloons and 1.15 mm with uncoated balloons (p < 0.001) . At 5 years, freedom from TLR was 77.5% for DCB and 59.1% for uncoated PTA. Furthermore, no difference in mortality was reported with a numerical advantage of DCB treated patients (82.7% survival after DCB vs 73.2% after uncoated PTA).
The CONSQUENT trial investigated the resveratrol paclitaxel coated SeQuentTM Please OTW balloon (B.Braun, Germany). 153 patients with symptomatic PAD in femoro-popliteal lesions were randomized either to DCB or uncoated PTA. The mean lesion length was 13.2 cm with target lesion total occlusions in 26.1% of all patients. LLL at 6 months was significantly reduced in the DCB group as compared to the POBA group (0.35 mm vs. 0.72 mm, p = 0.006) .
A patient level meta-analysis including patients from Thunder, FemPac, Pacifier and CONSEQUENT trials revealed no significant difference from all-cause death at 2 years (log rank p = 0.54). Causes of death were well balanced between the groups with no pattern or trend in favour of any specific causes in the PBC group. Logistic regression revealed that treatment groups (controls or PBC) were not a predictor of 2-year mortality. The only predictor for mortality was patient age ≥ 75 years .
Below the knee
Restenosis rate in infra-tibial artery disease range from 42 % at 12 months for short lesions till 69 % at 3 months for a lesion length of > 18 cm. Initial non-randomised series and RCT indicated favorable outcomes with the In.Pact Amphirion DCB in this challenging scenario , . However, the IN.PACT DEEP RCT using the same device could not confirm these findings. Compared to PTA, no biological effect was seen in terms of LLL or TLR. Furthermore, there was a statistical trend in terms of major amputations at one year (8.8 vs. 3.6%; p=0.08) . Several factors have been discussed as reasons for this findings like different protocols for wound care in higher Rutherford classes, device-specific issues like drug loss on the way to the lesion, or simply (by chance) the best ever reported PTA outcomes in such a patient population. Since conflicting data exist on the impact of DCB below the knee, the results of ongoing trials including improved devices and protocols for wound care should be awaited.
DCB have a number of advantages over standard angioplasty and stent technologies including (i) the potential for homogeneous drug delivery to the vessel wall which is not accomplished using DES, (ii) an immediate drug release without the use of a polymer which can induce chronic inflammation and late thrombosis as observed with some DES, (iii) the option of using balloon catheters alone or in combination with a bare metal stent, (iv) no foreign object such as DES left behind in the body, (v) the potential of reducing antiplatelet therapy, and (vi) lower restenosis rates in target peripheral (and also coronary) arteries compared to conventional treatment. DCB can also be applied in cases where stent implantation is not desirable or possible, such as femoro-popliteal arteries. Thus, the concept of using a balloon catheter to directly deliver an anti-restenotic drug at the site of injury is of paramount interest and convincing. The extension of endovascular therapy to longer and more demanding lesions might also increase the demand for a method that reduces the risk of restenosis without irreversibly modifying the structure of the vessel.
The results from randomized controlled coronary clinical trials consistently show that paclitaxel in a matrix of soluble additive coated on balloons reduces neointimal formation, as well as LLL, restenosis, and repeat revascularisation in patients with complex coronary artery lesions. It seems that in the coronary circulation, paclitaxel coated balloon angioplasty holds the greatest promise for lesions in which stent deployment is not desirable or technically challenging (e.g., in-stent restenosis, long and distal lesions, very angulated segments, small vessels or bifurcation lesions).
Obviously, the paclitaxel formulation is important since some balloon catheters coated with the same or a similar dose of paclitaxel failed to show efficacy in animal experiments and clinical trials. We will still have to learn much more about the benefits but also the limitations of this technology. Even when the same drugs were taken, there is considerable difference in the delivery efficacy of each balloon. It has to be pointed out that DCB may be different even if the same drug and dose has been chosen. Therefore, there is a clear need for randomized clinical trials in different indications and even more for preclinical studies on efficacy and tolerance and subsequent clinical trials on different coatings. As with DES, one cannot assume a class effect for DCB.
Data from randomized clinical trials identified the treatment of coronary ISR as accepted indication for DCB (ESC guidelines class 1 level A recommendation). In coronary de novo disease, a strategy of DCB only angioplasty fulfills the requirements of the concept of ‘leaving nothing behind’.
In the meantime, DCB were about to become the standard therapy for the transfemoral region. However, a meta-analysis was published in December 2018 reporting increased mortality 2 or more years after treatment with paclitaxel-coated stents or balloons . Although this study level analysis has considerable methodological flaws and a conclusive patho-mechanism does not exist, it has led to massive uncertainty, as a result of which many patients no longer receive the therapy that is most appropriate for them. The criticism includes a selection bias in longer follow-up (e.g. exclusion of trials with numerically lower mortality in the DCB group) and accounting for wrong numbers (e.g. Thunder trial at 5 years). The published event rates were divided by the intention to treat number of patients, which does not sufficiently consider the influence of cross-over between groups and lost to follow up. In the past, such an approach has raised doubts about the safety of coronary sirolimus DES , involving vigilance by regulatory authorities, which could not be reproduced in patient level analyses . Alternative explanations for the mortality findings may include interactions with the health care system and management of comorbidities. E.g. in the IN.PACT SFA trial compliance with scheduled follow-up visits was significantly higher in PTA patients. Visit compliance among DCB patients was higher for patients who survived versus died. PTA patients exhibited significantly higher rates of dual antiplatelet therapy compared to DCB treated patients . In the meantime, no evidence of increased mortality could be found in large registries with thousands of patients real world (7,8). Furthermore, a recent meta-analysis for the coronary use of paclitaxel DCB even shows a lower mortality after 3 years . Therefore, this therapy, which significantly improves the quality of life, should no longer be withheld from patients .
Drug coated balloons (DCB) have no class effect. Data from randomized clinical trials identified the treatment of coronary ISR as accepted indication for DCB (ESC guidelines class 1 level A recommendation). In coronary de novo disease, a strategy of DCB only angioplasty fulfills the requirements of the concept of ‘leaving nothing behind’. Furthermore, DCB may be the preferred treatment option in femoro-popliteal arteries independent from the ongoing mortality discussion.
Francesco Prati, Flavio Giuseppe Biccirè
Updated on May 13, 2021
Soo-Teik Lim, Tian-Hai Koh
Updated on February 1, 2018
Scot Garg, Sharmaine Thirunavukarasu, Raffaele Piccolo, Patrick W. Serruys, Stephan Windecker
Updated on January 23, 2018
Kai Ninomiya, Scot Garg, Alexandre A. Abizaid, Ron Waksman, Ashok Seth, Joanna Wykrzykowska, John A. Ormiston, Patrick W. Serruys, Yoshinobu Onuma
Updated on September 30, 2022