Near-infrared spectroscopy
Addendum: Prospect II & Prospect Absorb trials
Published on March 9, 2021
A report from Piera Capranzano, Rodney De Palma
The PROSPECT II natural history study aimed to assess the relationship between high-risk characteristics of untreated non-culprit lesions (NCLs) and long-term clinical outcomes. Among patients (n=898) with recent myocardial infarction (MI) NCLs were prospectively identified by intravascular ultrasound (IVUS) and their lipid content was assessed by near-infrared spectroscopy (NIRS). The intravascular imaging assessment was performed in the proximal 6-10 cm of the three coronary arteries after successful treatment of all flow-limiting lesions with contemporary drug-eluting stents. Main results of the PROSPECT II study can be summarized as follows1.
- At a median follow-up of 3.7 years, the rate of major adverse cardiovascular events (MACE, cardiac death, MI, unstable angina, or progressive angina) related to untreated NCLs was 8% vs. 4.6% of recurrent events related to treated culprit lesions.
- NIRS identified lipid-rich plaques that were responsible for future coronary events.
- In the multivariable analysis, plaque burden more than 70% and lipid core burden index (LCBI) >324 were independent predictors of MACE.
- The combination of lipid-rich plaque (LCBI >324) and large plaque burden identified vulnerable plaques that placed patients at especially high risk for future MACE.
The prophylactic treatment of high-risk plaques was investigated in the embedded PROSPECT ABSORB trial2. In this trial patients (n=182) with an angiographically nonobstructive stenosis not intended for PCI but with IVUS plaque burden of ≥65% were randomized to receive either ABSORB BVS + guideline-directed medical treatment (GDMT) or GDMT alone. Main results of the PROSPECT ABSORB study can be summarized as follows2.
- The primary effectiveness endpoint (powered) of IVUS-derived minimum lumen area at 25-month was significantly higher in the BVS vs. the GDMT alone group (6.9 vs. 3.0 mm2).
- The primary safety endpoint (nonpowered) of target lesion failure (cardiac death, target vessel-related MI, or clinically driven target lesion revascularization) at 24 months occurred in similar rates in the BVS and GDMT groups (4.3% vs. 4.5%).
- The secondary clinical effectiveness endpoint (nonpowered) of randomized lesion-related MACE at latest follow-up occurred in 4.3% the BVS vs. 10.7% in GDMT alone groups.
In conclusion, prophylactic treatment of high-risk non-flow limiting plaques appear to be beneficial in terms of expanding the lumen area and possibly in preventing lesion-related clinical events, warranting investigation in future adequately powered randomized trials.
1. Presented at TCT 2020
2. Stone GW et al. J Am Coll Cardiol 2020;76:2289-2301
Summary
Intracoronary near infrared spectroscopy (NIRS) is an optical imaging method for the identification of lipid core plaques (LCP) in the coronary arteries of patients undergoing coronary angiography. In 2019, on the basis of results from the Lipid-Rich Plaque Study the US FDA approved NIRS for the identification of high risk plaques and patients at increased risk of coronary events.
NIRS has been combined with traditional grey-scale intravascular ultrasound (IVUS) in a hybrid catheter that provides co-registered architectural and compositional plaque characterization data. The main advantage of NIRS over other plaque characterisation methods is its ability to provide an automated, direct identification of chemical composition, which is the primary use of spectroscopy for other applications. Identification of lipid core plaque with NIRS has potential to improve the safety of stenting, including optimisation of length of vessel to stent, and identify lipid-core lesions at higher risk of distal embolization during stenting. NIRS by itself does not display structures, but the combination device with co-registered IVUS gives simultaneous and complementary structural and compositional information. The ability of NIRS to identify vulnerable plaques and vulnerable patients described above may facilitate implementation of strategies to prevent future coronary events.
Limitations of coronary angiography and importance of plaque composition: need for improved imaging techniques
Observations from several large-scale clinical studies have had substantial impact on the management of patients with coronary artery disease. , , , In aggregate, these studies emphasize that clinical decision-making in individual patients is complex and often surrounded by uncertainty. The clinician is faced daily with challenging decisions regarding revascularisation strategies (whether percutaneous coronary intervention (PCI) should be offered versus bypass surgery) and important procedural issues regarding performance of PCI (e.g., identification of which lesions to treat, length of vessel to treat, optimal stent deployment, risk of distal embolisation and periprocedural myocardial infarction (MI), etc.).
Unfortunately, coronary angiography alone fails to provide adequate information for such decisions. Although a crucial tool to delineate the gross presence of disease and quantify the degree of stenosis, angiography underestimates the magnitude of atherosclerotic burden, particularly in earlier stage disease during which positive vascular remodeling may allow “normal” lumen calibre despite substantial vascular wall plaque. Importantly, angiography also has significant limitations in the precise delineation of plaque architecture and does not provide data on plaque composition or its biological activity.
Plaque composition has important clinical implications (Table 1): the presence of a lipid core specifically appears to be a critical determinant of plaque instability. , , , , Whereas fibrous plaques are thought to be more stable, lipid core plaques (LCP) are implicated in more rapid progression of coronary atherosclerosis, plaque rupture resulting in acute coronary syndromes and sudden cardiac death, as well as distal embolisation complications during PCI., , , , , The recognition of the ubiquity of substantial but non-flow limiting lesions that may be at high risk for subsequent plaque rupture has resulted in a paradigm shift in thinking about the pathophysiology of coronary artery disease, with the focus no longer solely on the degree of arterial luminal narrowing. This growing need for more information about coronary atherosclerosis in order to identify patients and lesions at risk of complications during PCI, and for future adverse cardiac events, has been the primary impetus for the development of novel intra-coronary imaging methods able to detect plaque composition, in particular the presence of a lipid core.
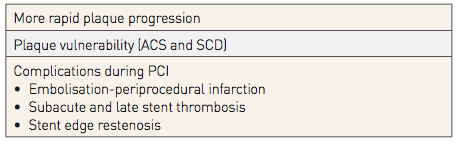
Table 1
Rationale for direct coronary imaging techniques
The ideal invasive tool for characterisation of coronary plaque should provide a complete roadmap of atherosclerotic burden throughout the coronary tree and in particular the morphological and compositional data for each plaque. Specific parameters should include:
- (1) extent of luminal stenosis;
- (2) lesion length;
- (3) coronary blood flow reserve through any given stenosis;
- (4) intramural plaque architecture including atheroma burden, eccentricity, and local vascular remodeling;
- (5) plaque composition, specifically lipid content;
- (6) fibrous cap thickness; and
- (7) presence of inflammation.
A comparison of the various intracoronary imaging techniques is provided in Table 2.
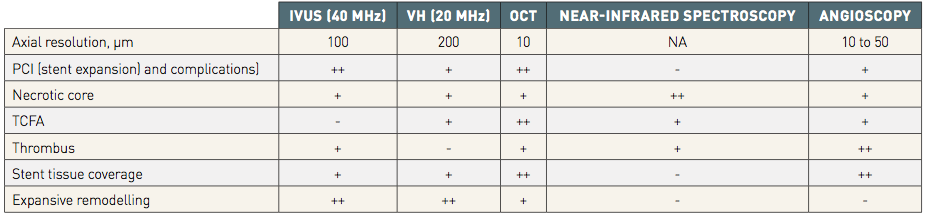
Table 2
++ Indicates excellence / + Good / + Possible / - Impossible.
Diffuse reflectance near infrared spectroscopy (NIRS)
Diffuse reflectance near-infrared spectroscopy (NIRS) is widely used in many fields to identify the chemical composition of unknown substances. Spectroscopy can be broadly defined as the measurement of the wavelength dependent interaction of electromagnetic radiation with matter. In diffuse reflectance NIRS, a sample of interest is irradiated with near-infrared light and a detector measures the proportion of diffusely reflected light returned as a function of wavelength. Two quite different processes determine the amount of light that returns to the detector – scattering and absorption.
- Scattering occurs when the path of the light is altered by the cellular and extracellular structures (that are larger than the wavelength of light) in the material.
- Absorption occurs when light energy is absorbed by chemical bonds of the constituent molecules. Absorbed light is mainly transformed into molecular vibrational energy in the form of oscillations of atoms within their chemical bonds.
NIRS is well-suited for detection of LCP in a coronary vessel tissue since it 1) can penetrate blood, 2) can penetrate several millimetres into the tissue, 3) can be done with an ultrafast scanning laser, overcoming the problem of cardiac motion, 4) is capable of acquiring the thousands of spatial measurements required to create an image of the artery, and 5) provides a positive and specific chemical measure of LCP (it does not rely on drop-out of signal for LCP detection as do intravascular ultrasound (IVUS) and optical coherence tomography (OCT)) since cholesterol has prominent features in the NIR region that can distinguish it from other tissue constituents such as collagen and calcium.
A single modality intravascular NIRS catheter system that uses diffuse reflectance spectroscopy was originally developed for invasive detection of LCP. Recognizing the need to provide structural imaging, a combined NIRS-IVUS catheter was subsequently introduced and approved by the FDA and has a CE mark. (Figure 1) This novel catheter allows more complete characterisation of coronary plaques, providing simultaneous and co-registered acquisition of their structural and compositional information.
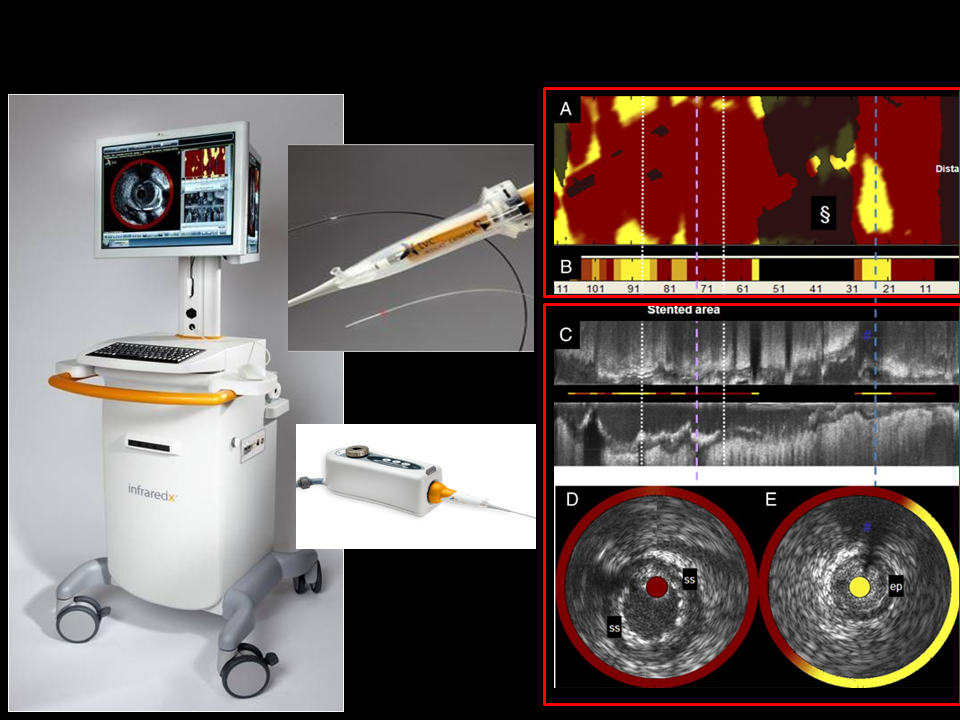
Figure 1
Combined NIRS-IVUS catheter (InfraReDx, Inc., Burlington, MA, USA) system components, including console (left panel), pullback and rotation (PBR) device (lower inset), catheter (upper inset) and images obtained (Chemogram[A], summary block chemogram [B], longitudinal IVUS pullback depicting a stented coronary segment [C], and cross-sectional IVUS (D, E) corresponding to dashed lines, with the chemogram data overlaid in a halo while the block chemogram colour value is portrayed in the central catheter artefact (yellow indicating a high probability of LCP).
In addition to the extensive established value of IVUS alone, the complementary nature of the NIRS compositional and IVUS structural information allows more complete characterization of coronary plaques. , , , Delineation of plaque architecture and composition has the potential to improve safety and long-term outcomes of PCI by: (1) Delineation of length of vessel to stent with adequate lesion coverage and optimal stent expansion; (2) Identification of large LCP, which are known to be at high risk of distal embolisation and periprocedural MI; (3) Detection of “vulnerable” LCP’s, those lesions posing greatest risk for future site-specific adverse events including acute coronary syndrome (ACS) and sudden death; and (4) Identification of vulnerable patients, defined as those patients who are at increased risk of future major adverse cardiovascular events (MACE). Owing to the recognized value of multimodality imaging techniques providing information on both structure and composition of coronary plaques, a combined NIRS and OCT catheter is now in development. The combined NIRS-OCT catheter will have clinical utility akin to the NIRS-IVUS device, but will have the added benefit of direct visualization and measurement of fibrous cap thickness, suspected to be a critical component of plaque stability. The visualization of fibrous cap thickness by the NIRS-OCT device will be facilitated by the higher spatial resolution of OCT compared to IVUS.
NIRS principles of operation
The NIRS-IVUS device is comprised of a scanning near-infrared laser and a fiber optic catheter similar in size and is now coupled with an IVUS catheter, and an automated pullback and rotation device. The NIRS-IVUS system consists of a 3.2 F rapid exchange catheter with an entry profile of 2.4F and a shaft profile of 3.6 F (Dualpro™ Catheter, Infraredx, A Nipro Company, Bedford, MA, USA) and a pullback device and console (Makoto Intravascular Imaging System™, Infraredx, A Nipro Company, Bedford, MA, USA). The 6F compatible NIRS-IVUS catheter can be inserted over a 0.014- inch guide wire whilst its passage through the lesion is facilitated by the hydrophilic coat present on the distal 50cm end. The original combined NIRS-IVUS device used a rotating IVUS transducer operating at 40 MHz. The 40 MHz IVUS technology has since been replaced with an IVUS transducer that emits an ultrasound signal at 50 MHz but collects and processes returning ultrasound signals ranging from 20 to 70 MHz. By doing so, the device provides excellent axial resolution while maintaining imaging at greater depths.
Hence, the current NIRS-IVUS catheter provides both NIRS information and high-definition IVUS images. Unlike OCT, there is no need to remove blood for NIRS-IVUS image acquisition. The catheter’s imaging core rotates at around 960 rpm with a maximum imaging length of 12 cm. The majority of the NIRS tissue information is obtained from a depth of 1 mm or less in the direction from the luminal surface toward the adventitia. Overall the system carries out more than 30,000 chemical measurements per 100 mm of imaged artery, which is consecutively scanned at a tissue depth of 1 mm over an area of 1-2 mm2. The radio-opaque marker on the NIRS catheter can be used to identify the location of the catheter and imaging element in relationship to target vessel fiduciary landmarks as detected by coronary angiography (e.g., stenoses of interest, branches, guide catheter, etc.) with the possibility to place a mark on the chemogram and annotate anatomical landmarks at the locations of acquisition of the NIRS spectra. (Table 3).
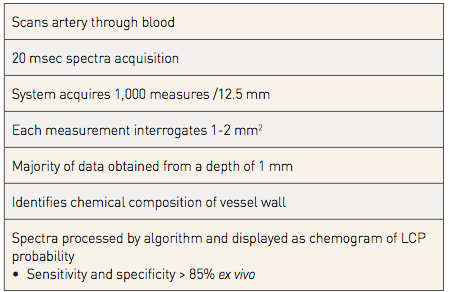
Table 3
A predictive algorithm calculates the probability that a LCP is present at each interrogated location in the artery. Upon completion of the imaging pullback, the data are immediately and automatically displayed on a two dimensional map of the vessel called a “chemogram.” The x-axis of the chemogram represents mm of pullback in the artery and the y-axis represents degrees of rotation; a colour scale from red to yellow indicates increasing algorithm probability that a LCP is present (Figure 2).
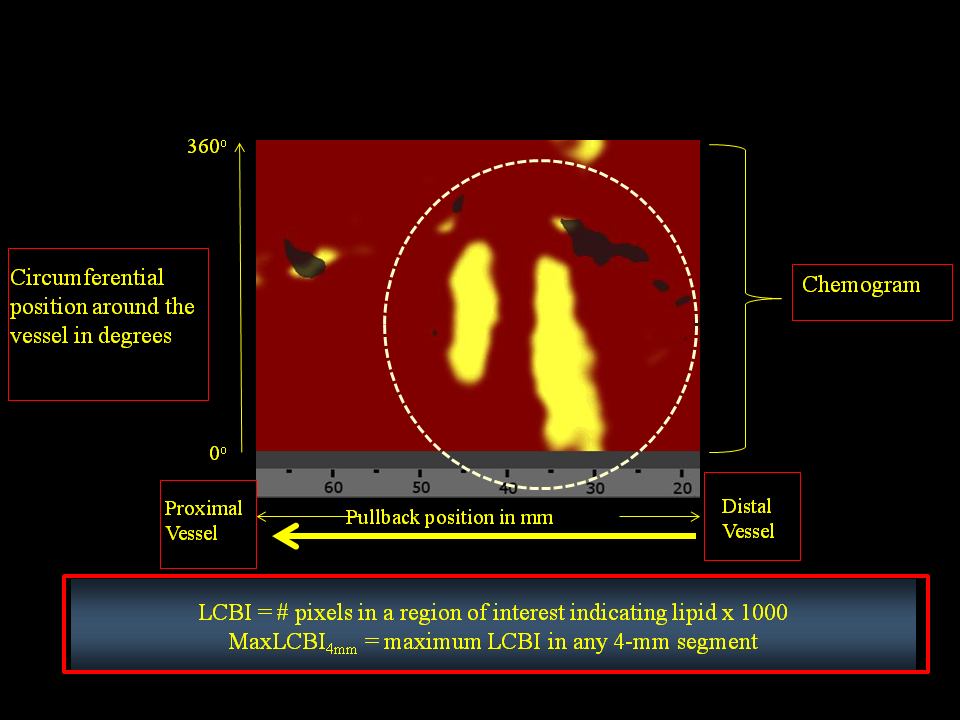
Figure 2
Illustration of methodology for determination of the maximum 4 mm sub-segment LCBI (maxLCBI4mm)?(A) The chemogram displays the NIRS findings obtained during rotation and pullback of the imaging element within the coronary artery of a patient. The x-axis represents mm of pullback, and the y-axis represents degrees of rotation from zero to 360. Yellow indicates a high probability that LCP is present at the interrogated site. (B) maxLCBI4mm is determined by defining the intervention region, computing LCBI for all 4 mm sub-segments within the intervention region, and identifying the maximum LCBI sub-segment. [Goldstein et al. Cir Cardiovasc Intervent]
The “block chemogram” provides a summary of the results for each 2 mm section of artery. The numerical value of each block in the block chemogram represents the 90th percentile of all pixel values in the corresponding 2 mm chemogram segment. The block chemogram is mapped to the same colour scale as the chemogram and the display uses four discreet colours to aid in the visual interpretation of the algorithm probability that a LCP is present in that 2 mm block (red: p<0.57, orange: 0.57≤p≤0.84, tan: 0.84≤p≤0.98, yellow: p>0.98). The lipid core burden index (LCBI) is provided as a quantitative summary metric of the LCP presence in the entire scanned region. LCBI is computed as the fraction of valid pixels within the scanned region that exceed an LCP probability of 0.6, multiplied by 1,000. Since the chemogram colour scale transitions from red to yellow near an LCP algorithm probability of 0.6, the LCBI can be viewed as a quantitative measure of the amount of yellow present on the chemogram. The NIRS software can also automatically identify the maximum LCBI in any 4-mm segment (maxLCBI4mm) within a larger region of interest. The maxLCBI4mm metric provides a quantitative estimate of focal lipid core size.
NIRS validation
To create an advanced algorithm ultimately applicable to interpretation of NIRS signals obtained in patients, initial calibration and validation studies were performed in intact and perfused human coronary artery autopsy specimens Figure 3. NIR spectrographic data were initially compared to the histology gold standard of a “LCP of interest”, defined as a fibroatheroma > 60 degrees in circumferential extent, >200 microns in thickness on a cross-sectional histologic specimen, and with a fibrous cap having a mean thickness of < 450 microns, a definition that was mandated by the FDA. Spectral similarity was prospectively analysed using common measures of multivariate distance and calibration model residual. In this rigorous, prospective study, an area under the receiver operating characteristic curve for NIRS detection of LCP of 0.83 (95% confidence interval, 0.70 to 0.93) was observed, satisfying the primary endpoint of the study. A clinical validation study with this catheter system was then performed, demonstrating that NIRS signals obtained in patients are spectrally similar to those obtained in autopsy specimens. Overall, results from these studies showed that the NIRS system is very accurate and valid for localised detection of LCP, as well as for determination of overall lipid burden of a scanned artery, in patients undergoing coronary angiography. (Figure 4)
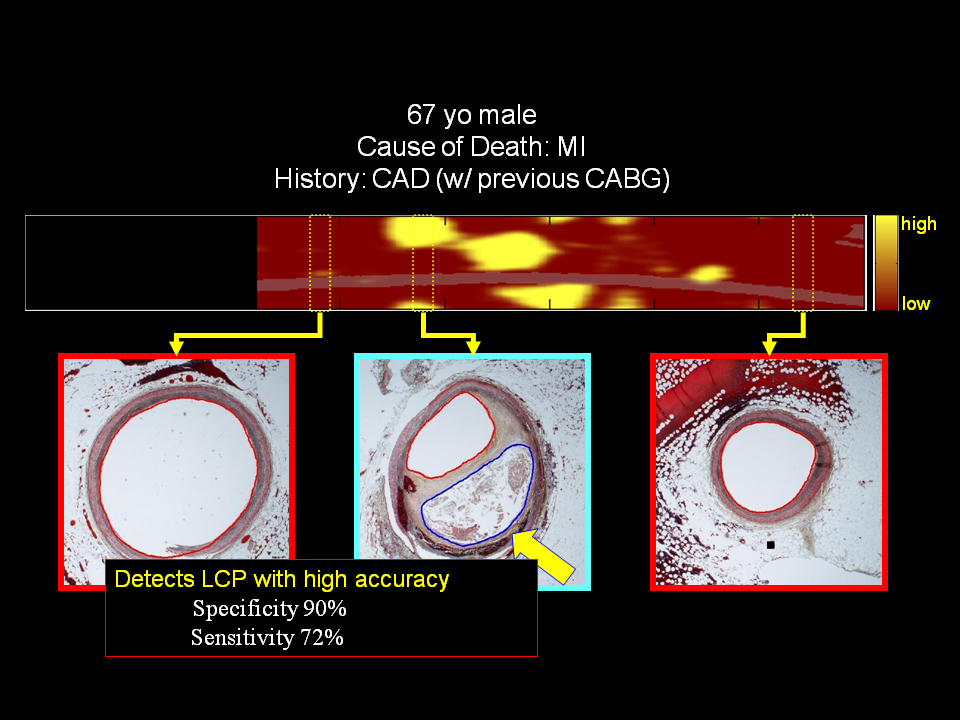
Figure 3
NIR spectrographic data compared to human coronary artery autopsy specimens. Block chemogram (upper panel) showing location of LCP and coronary autopsy cross-sections (lower images)
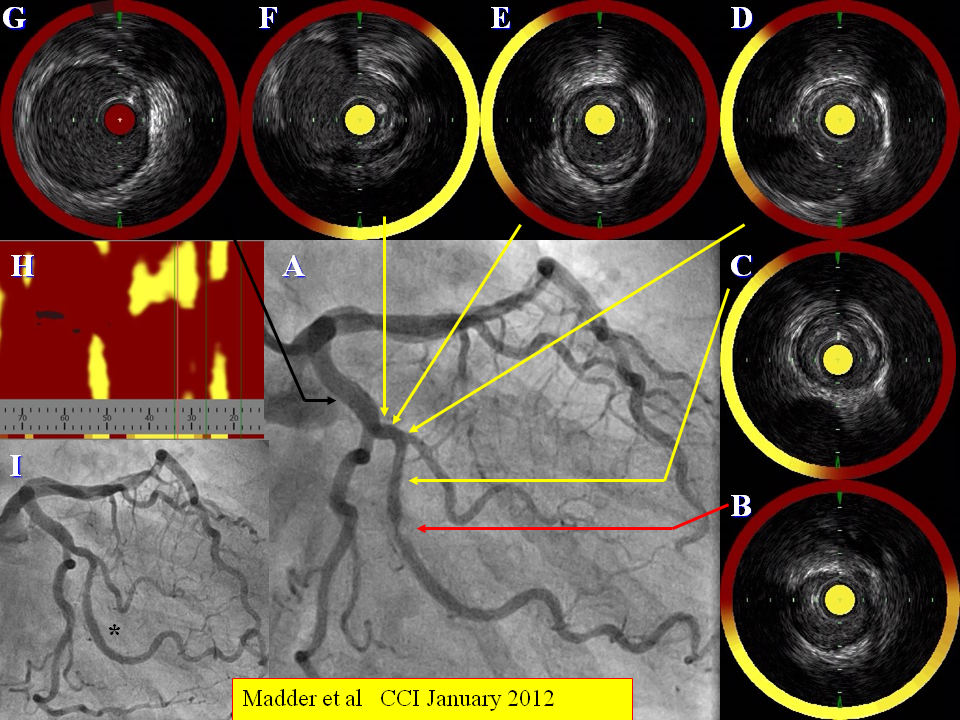
Figure 4
Target lesion composition in a patient undergoing coronary angiography (A). A target stenotic lesion in the obtuse marginal branch of the circumflex artery shows evidence of a large burden of LCP (B) as does the proximal and distal coronary segments at sites of minor angiographic disease (C-G). This is summarised in the chemogram (H). The stenosis is stented subsequently (I). From Madder et al CCI January 2012
Since the publication of these two original validation studies, several additional analyses comparing NIRS findings to histology have further validated the ability of NIRS to accurately detect coronary fibroatheroma. A study in which serial NIRS imaging was performed in hypercholesterolemic pigs demonstrated that NIRS-positive lesions, defined as an orange, tan, or yellow block on the NIRS block chemogram, detected histologically-proven fibroatheroma with a specificity of 95%. The high specificity of the NIRS block chemogram for detecting fibroatheroma has also been shown in a study of human coronary autopsy specimens, in which a yellow block on the NIRS block chemogram identified fibroatheroma with a specificity of 97%. Similar to the NIRS block chemogram, the NIRS LCBI metric is also capable of detecting histologically-proven fibroatheroma, as LCBI was previously shown to discriminate fibroatheroma in human coronary artery autopsy specimens with a c-statistic of 0.71. In this study, NIRS detection of fibroatheroma was shown to significantly improve by combining the NIRS LCBI with IVUS plaque burden, thereby supporting the importance of combined NIRS-IVUS findings. In another study comparing NIRS findings with histology in human coronary autopsy specimens, it was demonstrated that the maximum LCBI in 4mm (maxLCBI4mm) metric discriminated thin-capped fibroatheroma (TCFA) with a c-statistic of 0.84 and that a maxLCBI4mm ≥323 detected TCFA with a sensitivity of 80% and specificity of 85%.
NIRS comparisons with other imaging modalities
IVUS-Virtual Histology (VH) findings have been compared with NIRS findings. Larger coronary plaques, identified by greyscale IVUS, were more likely to be recognised as lipid core plaques (LCP) and as necrotic-core rich plaques by NIRS and VH, respectively; however, the correlation between NIRS and VH was poor. The fundamental differences in the principles of each technique, i.e. VH is based on a pattern classification of the backscattering ultrasound signal, whereas NIRS is based on near-infrared spectral signals, and their respective limitations should be taken into account in the interpretation of the differing results between NIRS and IVUS-VH. ,
A study comparing the detection of LCP by OCT and NIRS has also been performed. Among 184 paired cross-sectional images analyzed by both OCT and NIRS in this study, there was a significant but modest correlation between lipid arc defined by NIRS and lipid arc defined by OCT. However, the overall agreement between NIRS detection of LCP and OCT detection of LCP was suboptimal. Using NIRS as the reference standard, OCT detected LCP with good sensitivity (85.5%), but weak specificity (69.7%). The positive predictive value of OCT for detection of LCP was only 58.9% and the investigators observed that macrophage clusters were significantly associated with false positive LCP results by OCT.
Importance of Direct Coronary Imaging to Guide Optimal PCI
To fully appreciate the potential clinical value of NIRS-IVUS imaging, it is essential first to consider the growing body of evidence demonstrating that compared to angiographic guidance alone, PCI performed employing intracoronary imaging achieves superior outcomes. Selection and placement of a coronary stent on the basis of angiographic information alone has been associated with increased stent complications and worse outcomes compared to PCI guided by IVUS. , , , , . IVUS-guided PCI has been shown to reduce stent complications (dissection, early or late stent thrombosis, or edge restenosis), geographical miss in stent placement (stent ends in an area with high plaque burden), and incomplete stent expansion or malapposition. A meta-analysis encompassing 11 studies in 19,619 patients documented the superiority of direct coronary imaging to facilitate optimal performance of PCI compared with angiography-guidance. Further, the recent DAPT DES study showed IVUS guidance changed the procedure in >75% cases, resulting in longer, more appropriately sized stents. In the era of second-generation drug-eluting stents, there have now been multiple randomized controlled trials comparing IVUS-guidance versus angiography-guidance during PCI. A meta-analysis of patient-level data from three of these randomized trials demonstrated that compared to angiographic-guidance, PCI performed with IVUS-guidance was associated with a 64% relative risk reduction in the composite endpoint of cardiac death, myocardial infarction, and stent thrombosis at 1 year. In this meta-analysis, a significant reduction in myocardial infarction was observed when IVUS was used to guide PCI.
In aggregate, these observations emphasizing the clinical benefits of the morphological data provided by IVUS offer the basis for consideration of the value provided by NIRS-IVUS, a multi-modality catheter that provides accurate simultaneously obtained co-registered data on plaque composition as well as architecture. Therefore this multi-modality catheter may provide very powerful data to improve safety and long-term outcome of PCI (Table 4) by:
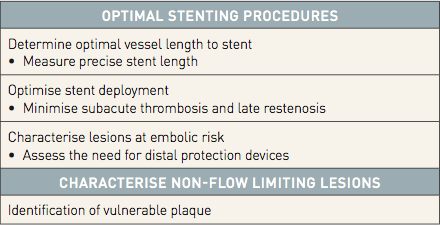
Table 4
- (1) Identification of large LCP, which are known to be at high risk of distal embolization and peri-procedural MI.
- (2) Optimisation of PCI, in terms of delineation of length of vessel to stent with adequate lesion coverage and optimal stent expansion.
- (3) Identification of vulnerable patients.
- (4) Identification of vulnerable plaques.
Prevention of periprocedural MI
Periprocedural MI is associated with short and long-term adverse outcomes. In particular, embolisation of the lipid core of stenotic plaques during PCI has been demonstrated as an important cause of angiographic no-reflow and periprocedural MI even in absence of intra-coronary thrombus. Since periprocedural MI can prolong hospital stay and is an impediment to more frequent performance of stenting in a less costly outpatient setting, pre-PCI identification of such plaques at high risk of embolisation might eventually lead to the development of preventive strategies, enhancing therefore the safety, efficacy, and cost-effectiveness of stenting. , , , , , , , ,
NIRS appears to be an effective technique for identification of LCP lesions at embolic risk. Prior evidence suggested, for example, that large LCP identified by NIRS might be associated with distal embolisation and periprocedural MI. (Figure 5, Figure 6). The COLOR Registry, a prospective observational study of patients undergoing NIRS prior to PCI, provided additional evidence of this capability of NIRS. In this study the extent of LCP in the treated region was calculated as the maxLCBI 4mm and showed that a periprocedural MI developed in 50% of patients with a maxLCBI4mm of ≥500 (22.6% of the lesions) and in only 4.2 % of patients with a maxLCBI4mm below this threshold (p=0.0002) (Figure 6). In the CANARY (Coronary Assessment by Near-infrared of Atherosclerotic Rupture-prone Yellow) trial, the optimal maxLCBI4mm target lesion threshold for predicting a periprocedural MI was a maxLBCI4mm of 388, with a c-statistic of 0.63 (IQR 0.5-0.77). In this trial, the predictive value of the maxLBCI4mm metric was strengthened by considering the degree of plaque burden at the target site. Post-PCI disappearance of “yellow”, which is common after stent placement, may in some cases reflect downstream embolisation of LCP contents (Figure 5).
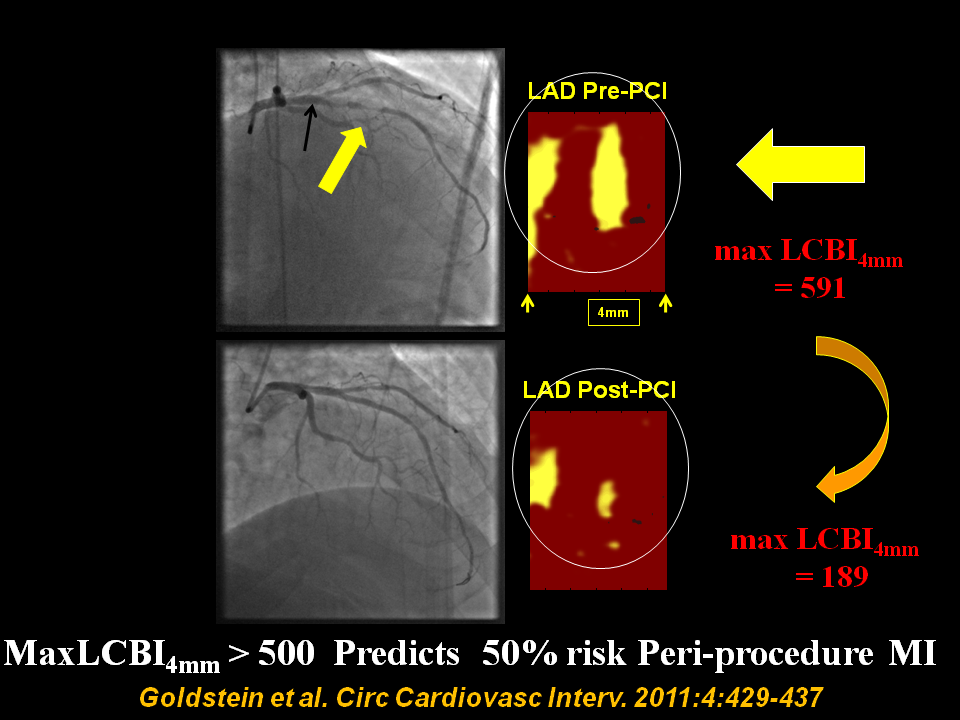
Figure 5
Pre and post percutaneous coronary intervention (PCI) angiogram frames and chemograms for a patient experiencing a periprocedural myocardial infarction (MI)?Pre- and post-percutaneous coronary intervention (PCI) angiogram frames and chemograms for a patient having a periprocedural myocardial infarction (MI). The yellow and black arrows indicate the location on the angiogram of the proximal and distal boundaries of the PCI location and correspond to the boundaries of the chemogram segment. Pre-PCI maxLCBI4 mm was 591 at the region, indicated by the 4-mm mark. The post-PCI chemogram shows substantial reduction in lipid-core plaque (maxLCBI4 mm reduced to 189 in the matched region). LCBI indicates lipid-core burden index; LAD, left anterior descending artery. From Goldstein et al. Circ Cardiovasc Interv. 2011:4:429-437
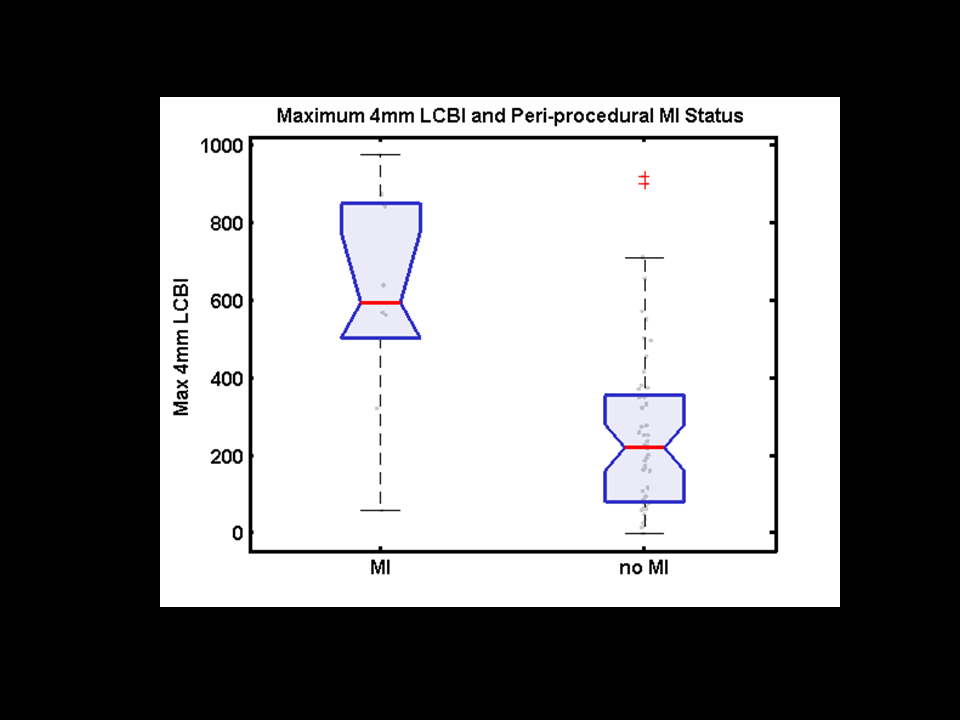
Figure 6
Box plot of maxLCBI4 mm grouped by occurrence of periprocedural myocardial infarction (MI). Black circles show the actual data points. Boxes have lines at the lower quartile, median (center line), and upper quartile values. Whiskers extend 1.5× the group interquartile range. Outliers (crosses) are data with values beyond the ends of the whiskers. Box notches indicate a robust estimate of the uncertainty about the medians, and lack of overlap of the notches visually shows that the 2 group medians differ at the 5% significance level. LCBI indicates lipid-core burden index. Goldstein et al. Circ Cardiovasc Interv. 2011:4:429-437
The knowledge that dilatation of a stenosis containing a large LCP identified by NIRS may carry an increased risk of causing a periprocedural MI indicates the need to develop preventive strategies, although no preventative therapies have been proven successful to date. The use of an embolic protection device (EPD) to prevent distal embolisation of plaque contents, already used in PCI of vein grafts and carotid arteries, is a particularly promising approach. Although prior studies of the use of EPDs during stenting of lesions in the native coronary arteries failed to show clinical benefit, these studies did not characterise, by intra-coronary imaging, the types of plaques most likely to embolise and therefore to benefit from the use of an EPD. The potential ability of NIRS-guided use of an EPD to prevent periprocedural MI has been preliminarily tested in the CANARY trial, which prospectively randomised patients undergoing PCI of a target lesion having a maxLCBI4mm ≥600 to either PCI with a distal EPD or to PCI alone. Overall, 21/85 (25%) patients in this trial had a periprocedural MI. Although those suffering periprocedural MI had a higher lipid core burden than those who did not experience an MI, this difference was not of statistical significance. However, in this small cohort with this particular EPD there was no difference in periprocedural MI between PCI with EPD compared to PCI alone. Additional studies evaluating the efficacy of EPD when performing PCI on NIRS-detected large LCP are needed. Novel approaches to reducing the risk of periprocedural MI, such as extracting the lipid content of culprit lesions prior to stent placement, require further study.
Optimisation of PCI
Angiography often shows diffuse irregularities and is known to grossly underestimate the extent of disease, even when the vessel segments appear “normal”. Conversely, IVUS imaging commonly confirms that sites with normal reference diameters may have extensive plaque with expansive remodeling, thereby fully delineating the length of plaque burden. Thus, the IVUS component of the NIRS-IVUS catheter delineates plaque burden, and documents optimal stent expansion and edge dissections after PCI. The value-added of NIRS is to delineate the length of the lesion composed of LCP, which may be critical to assist in decisions regarding the location and length of artery to be stented. Published clinical cases (Figure 7) document that placement of the ends of a stent over a LCP could result in a high frequency of stent thrombosis. Further highlighting the potential importance of identifying LCP in the stent margins, a recent study by Ino et al. demonstrated that uncovered LCP detected by OCT in the 5-mm margins outside the stent edges was associated with a significantly increased risk of edge restenosis during follow up.
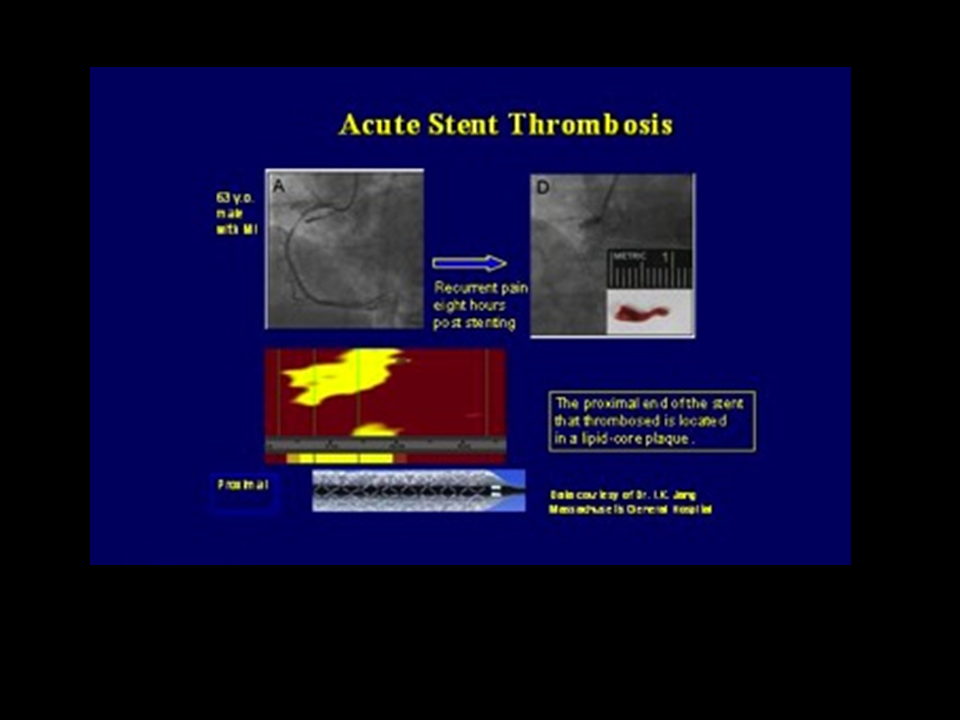
Figure 7
Acute stent thrombosis of a DES edge implanted in a not fully covered LCP. [Jang Circ Img]
Employing NIRS, Dixon et al demonstrated that in 16% of lesions LCP extended beyond the angiographic margins of the target lesion. A subsequent study employing NIRS-IVUS documented angiographic underestimation of lesion length in nearly 80% of cases (Figure 8). Thus, NIRS-IVUS may help to avoid placement of the ends of a stent in a LCP by accurately and precisely guiding full coverage of LCP; employing guidance by NIRS-IVUS, longer stents can be chosen to extend into vessel free of LCP or conversely the choice of a shorter stent could be supported by the absence of LCP in a given landing zone. Long-term studies are required to determine whether NIRS-informed stent length decisions result in better clinical outcomes.
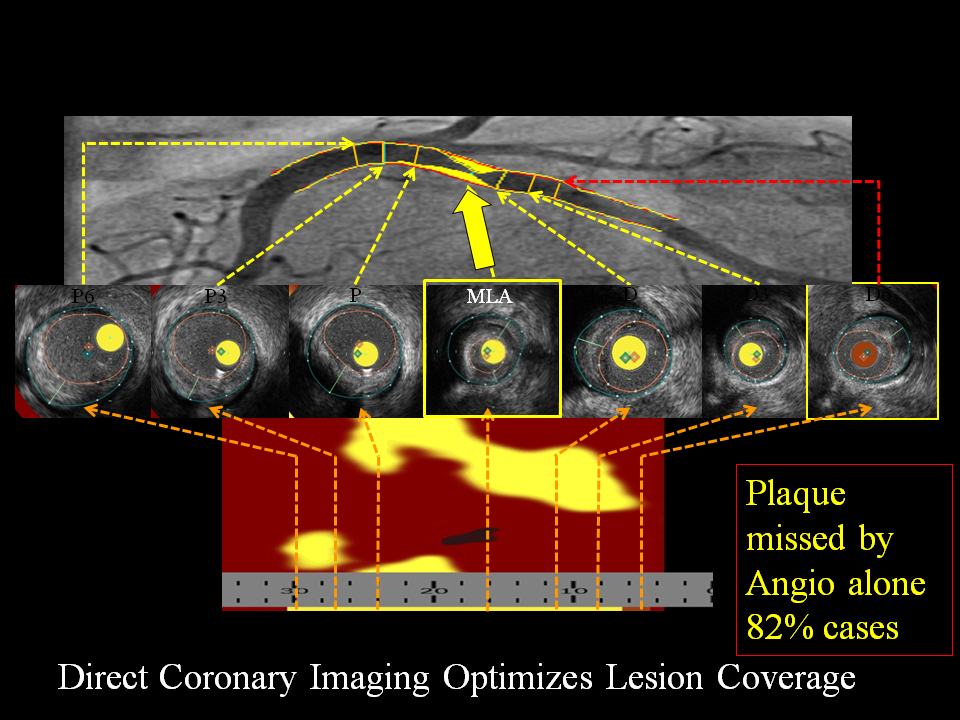
Figure 8
Use of NIRS to determine length of vessel to stent? Severe circumflex stenosis with corresponding chemogram documenting LCP. Contiguous proximal and distal plaque, though not severely narrowed, also contains LCP (P and D).
Identification of vulnerable patients
While the identification of vulnerable plaques has been a recognized goal since the introduction of the concept in 1989, identification of vulnerable patients has become of increasing importance with the appearance of novel and expensive pharmaceutical agents such as PCSK9 inhibitors and anti-inflammatory drugs. , , A vulnerable patient is defined as a patient at increased risk of cardiovascular events – identifying the site of a future culprit lesion is not necessary when identifying vulnerable patients.
Several small studies have demonstrated that NIRS findings within both target and non-target vessels can be used to identify vulnerable patients. In the first study to investigate the prognostic value of NIRS findings in non-culprit vessels, Oemrawsingh et al. found that patients with an LCBI in the non-culprit vessel at or above the median value of 43 for the study population had a four-fold increase in MACE during one year of follow up. The association between non-target vessel LCBI and an increased risk of subsequent MACE was also demonstrated in the ORACLE-NIRS registry. In this registry, the optimal non-target vessel LCBI threshold for predicting future MACE was an LCBI of 77.
Whereas LCBI, which provides an estimate of the total lipid burden within an imaged artery, has been associated with future cardiovascular risk, the maxLCBI4mm metric, which provides an estimate of focal lipid core size in a single lesion, has also been associated with future MACE risk. In a recent analysis by Schuurman et al, the largest maxLCBI4mm value within the non-culprit vessel was independently associated with future MACE risk over a median follow up of 4.1 years. In this study, there was a significant and continuous relationship between increasing maxLCBI4mm values and increasing MACE risk. Large LCP, defined as a maxLCBI4mm ≥400, within non-culprit segments of the culprit vessel have also been associated with MACE risk. When evaluating the association of various maxLCBI4mm thresholds with MACE risk, a dose response relationship was previously observed wherein a stepwise increase in LCP size was associated with significant increases in the risk of subsequent patient-level MACE.
The collective evidence from these small early studies suggested that NIRS imaging performed at non-culprit sites can be used to identify vulnerable patients at increased risk of subsequent patient-level MACE. This concept was recently confirmed by the results of the multicenter international Lipid-Rich Plaque Study, in which >1,200 patients undergoing PCI had NIRS-IVUS imaging performed in at least 2 coronary arteries at baseline. During 2 years of follow up, the baseline maxLCBI4mm detected by NIRS was associated with a significantly increased risk of patient-level MACE. These results provide definitive evidence that the NIRS instrument can be used to identify vulnerable patients, which led to the UD FDA approval for such and indication. Since the level of cholesterol in the blood predicts coronary events, it is not surprising that the accumulation of cholesterol in the wall of the coronary artery, which is an indication of disease and not solely a risk-factor, is a superior predictor.
Through detection of vulnerable patients, it is conceivable that NIRS could be utilized to select patients most likely to benefit from more aggressive secondary prevention strategies. It is also possible that NIRS might be applied to identify high-risk patients for inclusion in trials testing the efficacy of novel therapies aimed to reduce cardiovascular risk.
Identification of vulnerable plaque
Unstable plaques are thought to arise from “vulnerable” lesions at high risk of disruption. Retrospective autopsy studies of patients succumbing to acute MI and to sudden coronary death suggest several underlying histological culprit plaque morphologies, which thereby provide the foundation for the characterization of vulnerable plaques suspected to cause ACS events. The morphology suspected to be dangerous is an inflamed TCFA, characterized histologically by: 1.) thin fibrous cap (<65 µm), 2.) large necrotic lipid core, and 3.) enzymatically active macrophages near or within the fibrous cap.
Direct intra-coronary imaging has potential to detect vulnerable plaques responsible for subsequent events. IVUS provides useful information on plaque architecture, luminal stenosis and vessel remodeling and can be used to quantify the extent of lesion calcification and to assist in optimal stent deployment. By IVUS, complex unstable plaques are typically bulky, eccentric and exhibit features of disruption, such as ulceration, intimal flap and thrombus. , , , Culprit lesions in patients with ACS demonstrated more expansive/positive remodeling by IVUS than culprit lesions of patients with stable angina, suggesting that expansive remodeling might be associated with plaque vulnerability. , Characterisation of coronary plaques by greyscale IVUS is, however, not completely reliable, as, for example, echolucent and attenuated plaques have been variously related to high lipid content in some histological studies and to non-lipid component in other studies. , , , The PROSPECT Study demonstrated that certain plaque features obtained with IVUS-VH, such as plaque burden >70%, minimal luminal area < 4mm2, and the identification of VH-derived TCFA predicted the occurrence of subsequent events in 700 patients after an ACS at a median follow-up of 3.4 years. Similar findings have been demonstrated in subsequent IVUS studies. , Although the PROSPECT Study validated the concept that direct coronary imaging can prospectively characterise “vulnerable” lesions at risk of adverse events, the authors noted that the low specificity of the findings limit their clinical utility. It is also quite time-consuming to examine multiple IVUS cross-sections to identify increased plaque burden.
The identification of TCFAs as “suspected” vulnerable plaques provides a useful target that might be identified by novel intra-coronary imaging methods. Given the validated capabilities of NIRS for detection of LCP, fibroatheroma, and TCFA, it therefore followed that NIRS had the potential to detect “vulnerable” LCPs, those lesions posing greatest risk for future adverse events including ACS and sudden death. Madder et al. previously showed that the culprit lesion in patients with STEMI is by NIRS typically a large LCP (Figure 9); in particular, a maxLCBI4mm>400 accurately distinguished culprit from non-culprit segments within the artery and from the LCP–free autopsy histology segments (Figure 10). Similar results were replicated in a larger patient group presenting with STEMI in a multicenter study performed in the US and Sweden and have also been shown in patients presenting with non-STEMI and unstable angina. NIRS also allowed in vivo demonstration of large lipid cores in culprit segments present in a small group of sudden cardiac death survivors . It has also been shown that the target lesions responsible for ACS are in most cases large LCP’s and additionally patients with ACS commonly harbored remote, non-target lipid rich plaques. (Figure 11) , , , . Interestingly, in one study it was shown that the target lesion in patients with chronic stable angina was also commonly a LCP. Given that unstable versus stable angina is a clinical distinction, it may well be the case that some clinically “stable” patients harbor lipid-rich potentially unstable plaques that may be the precursor for a future ACS.
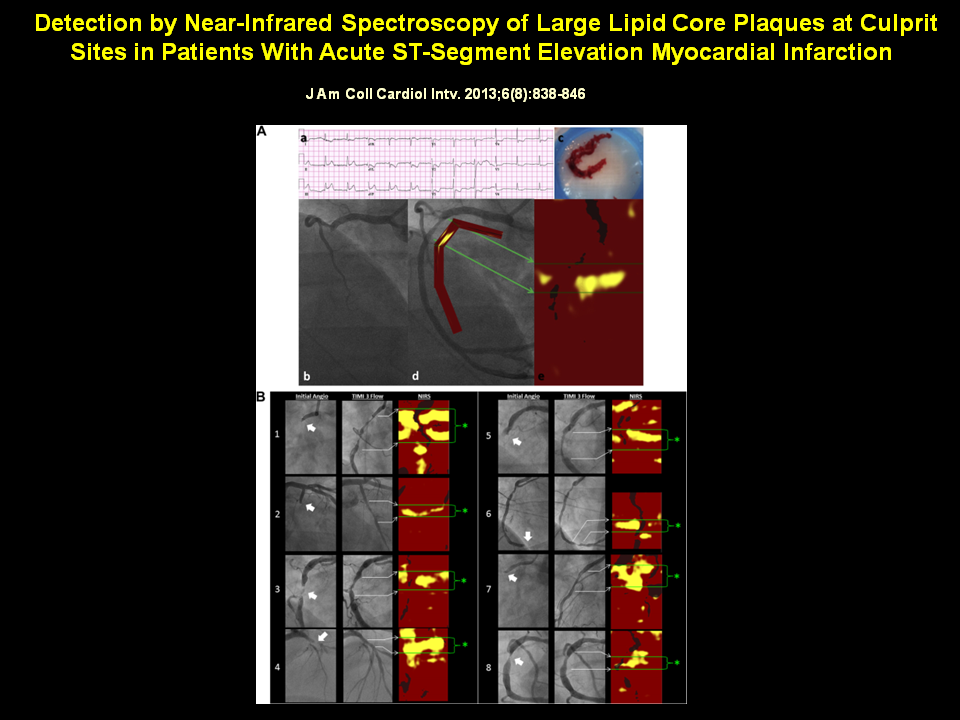
Figure 9
Angiographic and NIRS Findings in Acute STEMI
(A) A patient 56 years of age with acute chest pain and inferior-posterior injury (a) was referred for primary PCI. Angiography of the right coronary artery revealed complete occlusion (b). Aspiration yielded a thrombus characteristic of STEMI (c) and resulted in TIMI flow grade 3 (d). NIRS performed after TIMI flow grade 3 was established revealed a prominent, nearly circumferential LCP concentrated at the culprit site (e). (B) Angiographic and NIRS findings in 8 patients with STEMI. In each case, the initial angiogram demonstrated a culprit lesion (block arrow) with impaired flow. Following treatment, TIMI flow grade 3 is established. NIRS shows prominent signs of LCP at culprit sites (asterisks denote culprit segment located between green lines), often in a circumferential pattern. LCP = lipid core plaque; NIRS = near-infrared spectroscopy; PCI = percutaneous coronary intervention; STEMI = ST-segment elevation myocardial infarction; TIMI = Thrombolysis In Myocardial Infarction.
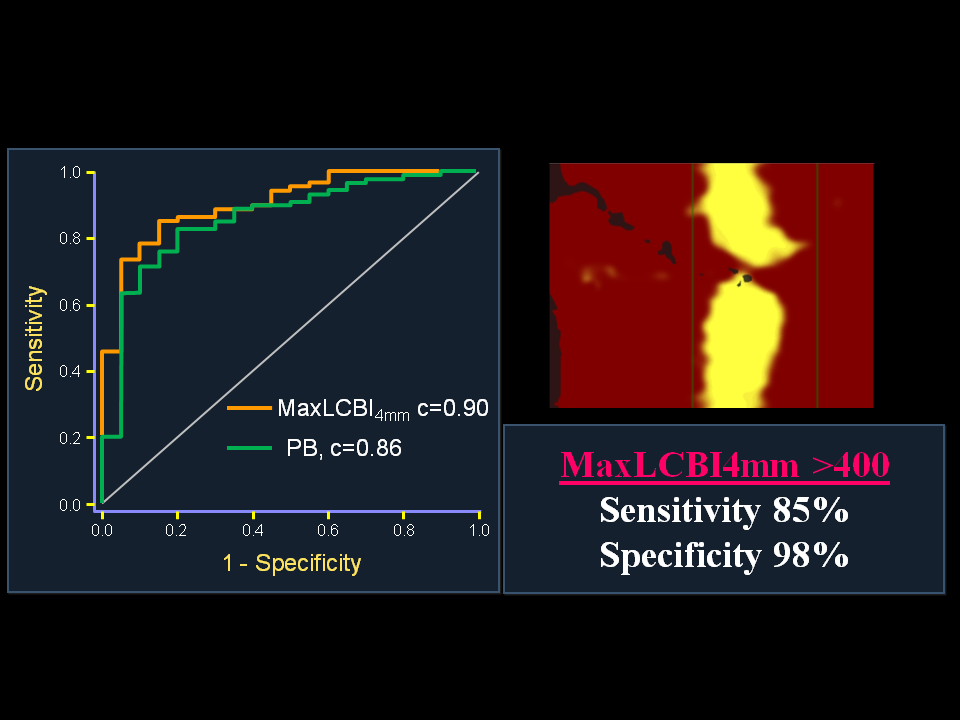
Figure 10
ROC Analysis for Detection of STEMI Culprit Segments by NIRS and IVUS
(A) The ability of NIRS and IVUS to distinguish culprit segments from nonculprit segments in the STEMI culprit vessel. MaxLCBI4mm(red), PB (green), and calcification (black) by IVUS are shown. MaxLCBI4mm was significantly more discriminatory than calcification (AUC: 0.90 vs. 0.72; p = 0.016) and performed similar to PB (AUC: 0.90 vs. 0.86; p = 0.44). (B) The ability of NIRS and IVUS to identify STEMI culprit segments when admixed with histology-negative autopsy specimens. MaxLCBI4mm(red), PB (green), and calcification (black) by IVUS are shown. MaxLCBI4mm was significantly more discriminatory at identifying STEMI culprit segments than were PB (AUC: 0.97 vs. 0.83; p = 0.015) or calcification (AUC: 0.97 vs. 0.79; p = 0.002). PB and calcification were not significantly different (p = 0.17). AUC = area under curve; ROC = receiver-operating curve; other abbreviations as in Figures 1, 2, and 4.
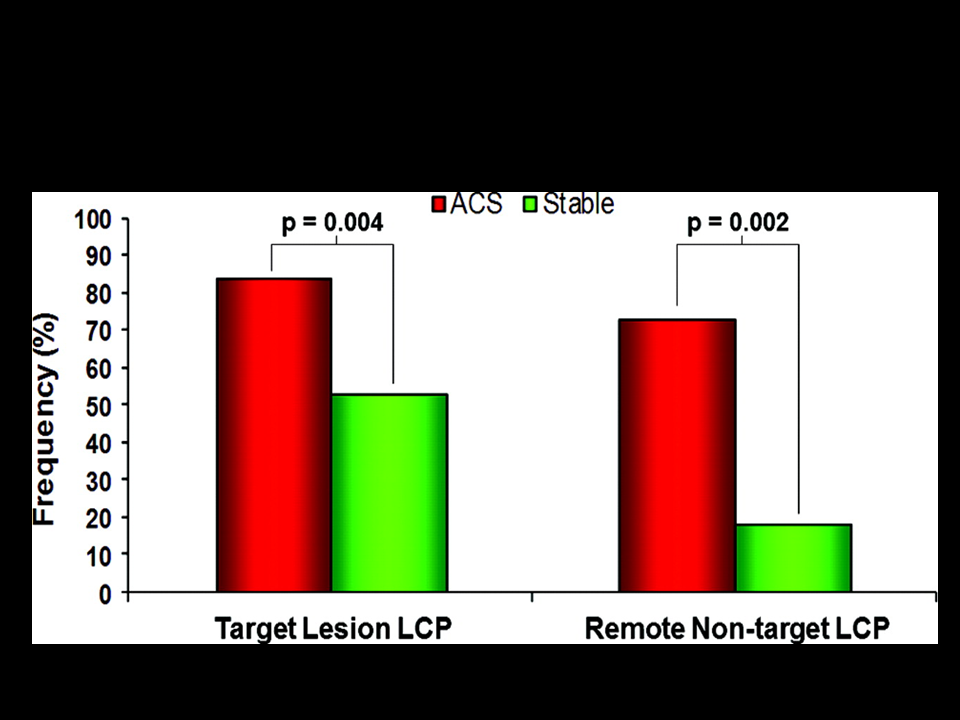
Figure 11
Target and remote, non-target LCP by NIRS in patients with ACS and stable angina.
The frequency of non-culprit LCP with the target vessel of patients with ACS and those with stable angina are shown. LCP=lipid-core plaques
In additional to testing the ability of NIRS to detect vulnerable patients, the Lipid-Rich Plaque Study also tested a co-primary hypotheses focused on whether NIRS could identify vulnerable plaques at increased risk of site-specific future MACE. In the Lipid-rich Plaque Study baseline images were segmented into 30-mm “Ware segments” for analysis of the NIRS-IVUS findings. Among the >1,200 patients in this study, there were a total of 5,755 Ware segments with NIRS-IVUS images at baseline that were followed for 2 years for the development of segment-specific MACE. This study found the in-segment maxLCBI4mm to be significantly associated with the risk of segment-specific MACE, with a HR of 1.45 (95% CI 1.30 – 1.60, p<0.0001) per 100 unit increase in maxLCBI4mm. It was also observed that a maxLCBI4mm >400 was associated with a >4-fold increase in the risk of segment-specific MACE (HR 4.22 [95% CI 2.39 – 7.45, p<0.0001]) during follow up. Thus, the Lipid-rich Plaque Study demonstrated, for the first time, that the NIRS maxLCBI4mm metric is capable of identifying vulnerable plaques at increased risk of triggering future site-specific events.
As evidence mounts for the greater propensity for rupture and more rapid progression when LCPs are present, the presence of LCPs in angiographically intermediate stenosis may support a strategy of preventive stenting of these vulnerable LCP’s. The essential first step to enable such a strategy is to test the hypothesis that such lesions are “at risk” for events. The prospective, multicentre PROSPECT II ABSORB Study (NCT02171065) performed in Scandinavia has completed enrollment of 900 ACS patients. NIRS-IVUS scanning was performed in all 3 coronary arteries post PCI of an index culprit lesion. The study is designed to determine the prognostic value of LCBI >400 evaluated over 2 years follow-up; it includes a randomised sub-study of trial of placement of BVS scaffolds in non-stenotic lesions with large plaque burden by IVUS with a stratified analysis based on amount of LCP detected by NIRS. Results of this study are expected in 2020.
Additional clinical uses
NIRS is likely to be helpful in the choice of more intensive lipid modification or anti-thrombotic therapies in certain patient subgroups. The presence of extensive LCP might indeed indicate the need for more intensive or different types of LDL-lowering therapies. NIRS has potential applicability in the assessment of novel anti-atherosclerotic medications, by providing a surrogate endpoint in plaque regression/stabilisation studies. In particular, the ability of NIRS to assess the lipid content of plaques may be a more effective means of identifying the beneficial effect of an agent than IVUS. The IBIS-3 (Integrated Biomarker and Imaging Study) assessed the effect of intensive rosuvastatin therapy on the content of necrotic core (IVUS-VH) and lipid-containing regions (NIRS) at 52 weeks in a non-intervened coronary artery. Although this study observed a neutral effect of high-dose rosuvastatin on LCBI in the overall study population, a significant reduction in LCBI was observed among patients with high baseline LCBI values.. The YELLOW trial recruited patients with multivessel CAD undergoing percutaneous coronary intervention. Patients received baseline assessment via NIRS and IVUS imaging, they were, then, randomised to a treatment of either rosuvastatin 40 mg daily or the standard-of-care lipid-lowering therapy. After 7 weeks of short term intensive statin therapy, a significant reduction in the lipid content was found when measured via maxLCBI4mm . In the ongoing YELLOW 2 trial (NCT01837823), all enrolled subjects will receive high-dose statin therapy, 40mg of rosuvastatin daily. Eight to twelve weeks after baseline imaging, the non-culprit yellow lesion will undergo staged intervention. The lipid rich lesion will be re-imaged to determine the effects of high-dose statin therapy in regards to reducing the lipid content and alterations in the plaque morphology; using NIRS and OCT/IVUS imaging techniques respectively. Similarly, the potential of other lipid lowering therapies such as lipid apheresis, HDL mimetic drugs, anti-inflammatory agents or new lipid lowering drugs, to decrease the lipid core volume or halt its progression are likely to be tested using this technology in the future.
Another potential future use could be to more fully inform the decision to perform PCI vs. CABG, based on the presence, patterns and extent of LCP, integrated with angiographic parameters in a “compositional syntax score”. Most notably changes could be envisioned in the determination of the presence of “three vessel disease” or patients that are “not candidates for PCI.” For example, it is reasonable to hypothesise that LCP-free, fibrotic stenoses in all three coronary vessels may in fact be better treated with stents. In contrast, diffuse lipid in all three vessels, either stenotic or non-stenotic, may turn out to be better treated by CABG.
Finally, at least one study has investigated NIRS findings in pre-existing coronary stents. In this study, it was proposed that combined NIRS-IVUS imaging might be able to identify neoatherosclerosis within previously implanted coronary stents and that such findings might be associated with an increased risk of subsequent stent failure. Validation studies of NIRS-IVUS imaging for the purpose of detecting neoatherosclerosis and potentially “vulnerable stents” at risk for late/very late stent thrombosis are needed.
Conclusions
- The degree of vessel narrowing caused by a plaque is the primary feature traditionally used to characterise coronary artery disease state. Traditional imaging techniques have not been able to provide accurate, easily obtainable information about plaque composition.
- The presence or absence of lipid core is likely to be one of the most important compositional parameters related to both the safety of stenting and the risk of rupture of a given plaque.
- NIRS-IVUS has a validated fundamental basis as a reliable method to measure lipid in a biological matrix composed of blood and tissue.
- Intracoronary NIRS-IVUS has been developed and rigorously validated as an accurate method for detection of lipid core plaque in patients undergoing coronary angiography.
- Early clinical use shows promise for the use of NIRS-IVUS to assist with common decisions in the catheterisation laboratory, such as identification of the risk of embolic infarction following balloon inflation for a stenosis and determination of length of artery to stent.
- The ability of NIRS-IVUS to predict slow- or no-reflow and associated periprocedural MI, suggests the need for preventive strategies, such as NIRS-guided use or an EPD.
- The results of the Lipid-rich Plaque study have demonstrated NIRS is capable of identifying vulnerable patients and vulnerable plaques. Since the FDA approval of NIRS for the identification of vulnerable plaques and vulnerable patients, the use of the technology is increasing worldwide.
Personal perspective - Ryan D. Madder
Unlike IVUS or OCT, which use the attenuation of signal to infer the presence of lipid, NIRS identifies LCP by detecting the presence of a spectroscopic signal that is highly specific for lipid. One of the major strengths of NIRS imaging for the detection of LCP is that NIRS was validated against the gold-standard of histology in multiple studies. The ability of NIRS to detect LCP at culprit lesions in ACS and to identify lesions at high risk of causing peri-procedural myocardial infarction has also been demonstrated. The Lipid-rich Plaque study has now convincingly demonstrated that NIRS can be used to identify vulnerable patients and vulnerable plaques. Finally, when considering the clinical utility of the multimodality NIRS-IVUS catheter, it should be noted that using IVUS to routinely guide PCI results in superior clinical outcomes compared to angiography alone.
- Waksman R, Di Mario C, Torguson R, Ali ZA, Singh V, Skinner WH, Artis AK, Cate TT, Powers E, Kim C, Regar E, Wong SC, Lewis S, Wykrzykowska J, Dube S, Kazziha S, van der Ent M, Sha P, Craig PE, Zou QZ, Kom P, Brewer HB, Garcia-Garcia HM. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: a prospective, cohort study. Lancet. 2019; 394:1629-37.
- Serruys PW, Morice MC, Kappetein AP , Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW, SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961-72.
- Boden WE, O’Rourke RA, Teo KK , Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS, COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503-16.
- Shaw LJ, Berman DS, Maron DJ , Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O’Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE, COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283-91.
- Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226-35.
- Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13-8.
- Yamagishi M, Terashima M, Awano K , Kijima M, Nakatani S, Daikoku S, Ito K, Yasumura Y, Miyatake K. Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol. 2000;35:106-11.
- Hong MK, Mintz GS, Lee CW, Lee BK, Yang TH, Kim YH, Song JM, Han KH, Kang DH, Cheong SS, Song JK, Kim JJ, Park SW, Park SJ. T. The site of plaque rupture in native coronary arteries: a three-vessel intravascular ultrasound analysis. J Am Coll Cardiol. 2005;46:261-5.
- Fujii K, Kobayashi Y, Mintz GS , Takebayashi H, Dangas G, Moussa I, Mehran R, Lansky AJ, Kreps E, Collins M, Colombo A, Stone GW, Leon MB, Moses JW. Intravascular ultrasound assessment of ulcerated ruptured plaques: a comparison of culprit and nonculprit lesions of patients with acute coronary syndromes and lesions in patients without acute coronary syndromes. Circulation. 2003;108:2473-8.
- Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O’Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343:915-22.
- Kolodgie FD, Burke AP, Farb , Gold HK, Yuan J, Narula J, Finn AV, Virmani R. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001;16:285-92.
- Heusch G, Kleinbongard P, Bose D, Levkau B, Haude M, Schulz R, Erbel R. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120:1822-36.
- Schultz CJ, Serruys PW, van der Ent M, Ligthart J, Mastik F, Garg S, Muller JE, Wilder MA, van de Steen AF, Regar E. First-in-man clinical use of combined near-infrared spectroscopy and intravascular ultrasound: a potential key to predict distal embolization and no-reflow?. J Am Coll Cardiol. 2010;56:314.
- Gardner CM, Tan H, Hull EL, Lisauskas JB, Sum ST, Meese TM, Jiang C, Madden SP, Caplan JD, Burke AP, Virmani R, Goldstein J, Muller JE. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. JACC Cardiovasc Imaging. 2008;1:638-48.
- Waxman S, Dixon SR, L’Allier P, Moses JW, Petersen JL, Cutlip D, Tardif JC, Nesto RW, Muller JE, Hendricks MJ, Sum ST, Gardner CM, Goldstein JA, Stone GW, Krucoff MW. In vivo validation of a catheter-based near-infrared spectroscopy system for detection of lipid core coronary plaques: initial results of the SPECTACL study. JACC Cardiovasc Imaging. 2009;2:858-68.
- Brugaletta S, Garcia-Garcia HM, Serruys PW , de Boer S, Ligthart J, Gomez-Lara J, Witberg K, Diletti R, Wykrzykowska J, van Geuns RJ, Schultz C, Regar E, Duckers HJ, van Mieghem N, de Jaegere P, Madden SP, Muller JE, van der Steen AF, van der Giessen WJ, Boersma E. NIRS and IVUS for characterization of atherosclerosis in patients undergoing coronary angiography. JACC Cardiovasc Imaging. 2011;4:647-55.
- Patel D, Hamamdzic D, Llano R, Patel D, Cheng L, Fenning RS, Bannan K, Wilensky RL. Subsequent development of fibroatheromas with inflamed fibrous caps can be predicted by intracoronary near-infrared spectroscopy. Arterioscler Thromb Vasc Biol. 2013;33:347-353.
- Kang SJ, Mintz GS, Pu J, Sum ST, Madden SP, Burke AP, Xu K, Goldstein JA, Stone GW, Muller JE, Virmani R, Maehara A. Combined IVUS and NIRS detection of fibroatheromas: Histopathologic validation in human coronary arteries. JACC Cardiovasc Imaging. 2015;8:184-94.
- Puri R, Madder RD, Madden SP, Sum ST, Wolski K, Muller JE, Andrews J, King KL, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE, Virmani R, Maehara A, Mintz GS, Nicholls SJ. Near-infrared spectroscopy enhances intravascular ultrasound assessment of vulnerable coronary plaque. Arterioscler Thromb Vasc Biol. 2015;35:2423-2431.
- Inaba S, Mintz GS, Burke AP, Stone GW, Virmani R, Matsumura M, Parvataneni R, Puri R, Nicholls SJ, Maehara A. Intravascular ultrasound and near-infrared spectroscopic characterization of thin-cap fibroatheroma. Am J Cardiol. 2017;119:372-378.
- Pu J, Mintz GS, Brilakis ES, Banerjee S, Abdel-Karim AR, Maini B, Biro S, Lee JB, Stone GW, Weisz G, Maehara A. In vivo characterization of coronary plaques: novel findings from comparing greyscale and virtual histology intravascular ultrasound and near-infrared spectroscopy. Eur Heart J. 2012;33:372-83.
- Di Vito L, Imola F, Gatto L, Romagnoli E, Limbruno U, Marco V, Picchi A, Micari A, Albertucci M, Prati F. Limitations of OCT in identifying and quantifying lipid components: an in vivo comparison study with IVUS-NIRS. EuroIntervention. 2017;13:303-311.
- Buccheri S, Fanchina G, Romano S, Puglisi S, Venuti G, D’Arrigo P, Francaviglia B, Scalia M, Condorelli A, Barbanti M, Capranzano P, Tamburino C, Capodanno D. Clinical outcomes following intravascular inaging-guided versus coronary angiography-guided percutaneous coronary intervention with stent implantation. J Am Coll Cardiol Intv. 2017;10:2488-98.
- Zhang Y, Farooq V, Garcia-Garcia HM, Bourantas CV, Tian N, Dong S, Li M, Yang S, Serruys PW, Chen SL. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012;8:855-65.
- Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Brodie BR, Stuckey TD, Mazzaferri EL Jr, Xu K, Parise H, Mehran R, Mintz GS, Stone GW. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129:463-70.
- Hong SJ, Kim BK, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Kang TS, Kang WC, Her AY, Kim YH, Hur SH, Hong BK, Kwon H, Jang Y, Hong MK. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation. The IVUS-XPL randomized clinical trial. JAMA. 2015;314:2155-2163.
- Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, Tian N, Lin S, Lu Q, Wu X, Li Q, Liu Z, Chen Y, Qian X, Wang J, Chai D, Chen C, Li X, Gogas BD, Pan T, Shan S, Ye F, Chen SL. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation. The ULTIMATE Trial. J Am Coll Cardiol. 2018;72:3126-37.
- Shin DH, Hong SJ, Mintz GS, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Hong MK. Effects of intravascular ultrasound-guided versus angiography-guided new-generation drug-eluting stent implantation. J Am Coll Cardiol. 2016;9:2232-9.
- Saucedo JF, Mehran R, Dangas G, Hong MK, Lansky A, Kent KM, Satler LF, Pichard AD, Stone GW, Leon MB. Long-term clinical events following creatine kinase--myocardial band isoenzyme elevation after successful coronary stenting. J Am Coll Cardiol. 2000;35:1134-41
- Prasad A, Singh M, Lerman A, Lennon RJ, Holmes DR, Jr. , Rihal CS. Isolated elevation in troponin T after percutaneous coronary intervention is associated with higher long-term mortality. J Am Coll Cardiol. 2006;48:1765-70.
- Selvanayagam JB, Porto I, Channon K et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation. 2005;111:1027-32.
- Mehran R, Dangas G, Mintz GS, Lansky AJ, Pichard AD, Satler LF, Kent KM, Stone GW, Leon MB. Atherosclerotic plaque burden and CK-MB enzyme elevation after coronary interventions : intravascular ultrasound study of 2256 patients. Circulation. 2000;101:604-10.
- Hong YJ, Mintz GS, Kim SW, Lee SY, Okabe T, Pichard AD, Satler LF, Waksman R, Kent KM, Suddath WO, Weissman NJ. Impact of plaque composition on cardiac troponin elevation after percutaneous coronary intervention: an ultrasound analysis. J Am Coll Cardiol Imaging. 2009;2:458-68.
- Kotani J, Nanto S, Mintz GS, Kitakaze M, Ohara T, Morozumi T, Nagata S, Hori M. Plaque gruel of atheromatous coronary lesion may contribute to the no-reflow phenomenon in patients with acute coronary syndrome. Circulation. 2002;106:1672-7.
- Kawamoto T, Okura H, Koyama Y, Toda I, Taguchi H, Tamita K, Yamamuro A, Yoshimura Y, Neishi Y, Toyota E, Yoshida K. The relationship between coronary plaque characteristics and small embolic particles during coronary stent implantation. J Am Coll Cardiol. 2007;50:1635-40.
- Uetani T, Amano T, Ando H, Yokoi K, Arai K, Kato M, Marui N, Nanki M, Matsubara T, Ishii H, Izawa H, Murohara T. The correlation between lipid volume in the target lesion, measured by integrated backscatter intravascular ultrasound, and post-procedural myocardial infarction in patients with elective stent implantation. Eur Heart J. 2008;29:1714-20.
- Goldstein JA, Grines C, Fischell T, Virmani R, Rizik D, Muller J, Dixon SR. Coronary embolization following balloon dilation of lipid-core plaques. JACC Cardiovascular imaging. 2009;2:1420-4.
- Goldstein JA, Maini B, Dixon SR, Brilakis ES, Grines CL, Rizik DG, Powers ER, Steinberg DH, Shunk KA, Weisz G, Moreno PR, Kini A, Sharma SK, Hendricks MJ, Sum ST, Madden SP, Muller JE, Stone GW, Kern MJ. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circulation Cardiovascular interventions. 2011;4:429-37.
- Stone GW, Maehara A, Muller JE, Rizik DG, Shunk KA, Ben-Yehuda O, Genereux P, Dressler O, Parvataneni R, Madden S, Shah P, Brilakis ES, Kini AS. Plaque characterization to inform the prediction and prevention of periprocedural myocardial infarction during percutaneous coronary intervention. JACC Cardiovasc Interv. 2015;8:927-36.
- Lee MS, Park SJ, Kandzari DE, Kirtane AJ, Fearon WF, Brilakis ES, Vermeersch P, Kim YH, Waksman R, Mehilli J, Mauri L, Stone GW. Saphenous vein graft intervention. JACC Cardiovasc Interv. 2011;4:831-43.
- Erlinge D, Harnek J, Goncalves I, Gotberg M, Muller JE, Madder RD. Coronary liposuction during percutaneous coronary intervention: evidence by near-infrared spectroscopy that aspiration reduces culprit lesion lipid content prior to stent placement. Eur Heart J Cardiovasc Imaging. 2015;16:316-24.
- Waxman S, Freilich MI, Suter MJ, Shishkov M, Bilazarian S, Virmani R, Bouma BE, Tearney GJ. A case of lipid core plaque progression and rupture at the edge of a coronary stent: elucidating the mechanisms of drug-eluting stent failure. Circulation Cardiovascular interventions. 2010;3:193-6.
- Ino Y, Kubo T, Matsuo Y, Yamaguchi T, Shiono Y, Shimamura K, Katayama Y, Nakamura T, Aoki H, Taruya A, Nishiguchi T, Satogami K, Yamano T, Kameyama T, Orii M, Ota S, Kuroi A, Kitabata H, Tanaka A, Hozumi T, Akasaka T. Optical coherence tomography predictors for edge restenosis after everlimus-eluting stent implantation. Circ Cardiovasc Interv. 2016;9:e004231.
- Dixon SR, Grines CL, Munir A, Madder RD, Safian RD, Hanzel GS, Pica MC, Goldstein JA. Analysis of target lesion length before coronary artery stenting using angiography and near-infrared spectroscopy versus angiography alone. Am J Cardiol. 2012;109:60-6.
- Hanson ID, Goldstein JA, Dixon SR, Stone GW. Comparison of coronary artery lesion length by NIRS-IVUS versus angiography alone. Coron Artery Dis. 2015;26:484-9.
- Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733-43.
- Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumub and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
- Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-99.
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119-1131.
- Oemrawsingh RM, Cheng JM, Garcia-Garcia HM, van Geuns RJ, de Boer SP, Simsek C, Kardys I, Lenzen MJ, van Domburg RT, Regar E, Serruys PW, Akkerhuis KM, Boersma E, ATHEROREMO-NIRS Investigators. Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease. J Am Coll Cardiol. 2014;64:2510-8.
- Danek BA, Karatasakis A, Karacsonyi J, Alame A, Resendes E, Kalsaria P, Nguyen-Trong PJ, Rangan BV, Roesle M, Abdullah S, Banerjee S, Brilakis ES. Long-term follow-up after near-infrared spectroscopy coronary imaging: Insights from the lipid cORe plaque association with CLinical events (ORACLE-NIRS) registry. Cardiovasc Revasc Med. 2017;18:177-181.
- Schuurman AS, Vroegindewey M, Kardys I, Oemrawsingh RM, Cheng JM, de Boer S, Garcia-Garcia HM, van Geuns RJ, Regar ES, Daemen J, van Mieghem NM, Serruys PW, Boersma E, Akkerhuis KM. Near-infrared spectroscopy-derived lipid core burden index predicts adverse cardiovascular outcome in patients with coronary artery disease during long-term follow-up. European Heart J. 2017;0:1-8.
- Madder RD, Husaini M, Davis AT, VanOosterhout S, Khan M, Wohns D, McNamara RF, Wolschleger K, Gribar J, Collins JS, Jacoby M, Decker JM, Hendricks M, Sum ST, Madden S, Ware JH, Muller JE. Large lipid-rich coronary plaques detected by near-infrared spectroscopy at non-stented sites in the target artery identify patients likely to experience future major adverse cardiovascular events. Eur Heart J Cardiovasc Imaging. 2016;17:393-399.
- Prati F, Arbustini E, Labellarte A, Dal Bello B, Sommariva L, Mallus MT, Pagano A, Boccanelli A. Correlation between high frequency intravascular ultrasound and histomorphology in human coronary arteries. Heart. 2001;85:567-70.
- Hiro T, Leung CY, De Guzman S, Caiozzo VJ, Farvid AR, Karimi H, Helfant RH, Tobis JM. Are soft echoes really soft?. Intravascular ultrasound assessment of mechanical properties in human atherosclerotic tissue. Am Heart J. 1997;133:1-7.
- Hara H, Tsunoda T, Moroi M, Kubota T, Kunimasa T, Shiba M, Wada M, Tsuji T, Iijima R, Nakajima R, Yoshitama T, Nakamura M. Ultrasound attenuation behind coronary atheroma without calcification: mechanism revealed by autopsy. Acute cardiac care. 2006;8:110-2.
- Yamada R, Okura H, Kume T, Neishi Y, Kawamoto T, Watanabe N, Toyota E, Yoshida K. Histological characteristics of plaque with ultrasonic attenuation: a comparison between intravascular ultrasound and histology. Journal of Cardiology. 2007;50:223-8.
- Calvert PA, Obaid DR, O’Sullivan M, Shapiro LM, McNab D, Densem CG, Schofield PM, Braganza D, Clarke SC, Ray KK, West NE, Bennett MR. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) study. J Am Coll Cardiol Imaging. 2011;4:894-901.
- Stone PH, Saito S, Takahashi S, Makita Y, Nakamura S, Kawasaki T, Takahashi A, Katsuki T, Nakamura S, Namiki A, Hirohata A, Matsumura T, Yamazaki S, Yokoi H, Tanaka S, Otsuji S, Yoshimachi F, Honye J, Harwood D, Reitman M, Coskun AU, Papafaklis MI, Feldman CL. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION study. Circulation. 2012;126:172-181.
- Madder RD, Goldstein JA, Madden SP, Puri R, Wolski K, Hendricks M, Sum ST, Kini A, Sharma S, Rizik D, Brilakis ES, Shunk KA, Petersen J, Weisz G, Virmani R, Nicholls SJ, Maehara A, Mintz GS, Stone GW, Muller JE. Detection by near-infrared spectroscopy of large lipid core plaques at culprit sites in patients with acute ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2013;6:838-46. http://www.ncbi.nlm.nih.gov/pubmed?. term=JACC Cardiovasc Interv. 2013;6:838-46.
- Madder RD, Puri R, Muller JE, Harnek J, Gotberg M, VanOosterhout S, Chi M, Wohns D, McNamara R, Wolski K, Madden S, Sidharta S, Andrews J, Nicholls SJ, Erlinge D. Confirmation of the intracoronary near-infrared spectroscopy threshold of lipid-rich plaques that underlie ST-segment elevation myocardial infarction. Arterioscler Thromb Vasc Biol. 2016;36:1010-1015.
- Madder RD, Husaini M, Davis AT, VanOosterhout S, Harnek J, Gotberg M, Erlinge D. Detection by near-infrared spectroscopy of large lipid cores at culprit sites in patients with non-ST-segment elevation myocardial infarction and unstable angina. Catheter Cardiovasc Interv. 2015;86:1014-1021.
- Madder RD, Wohns DH, Muller JE. Detection by intracoronary near-infrared spectroscopy of lipid core plaque at culprit sites in survivors of cardiac arrest. J Invasive Cardiol. 2014;26:78-9.
- Madder RD, Smith JL, Dixon SR, Goldstein JA. Composition of target lesions by near-infrared spectroscopy in patients with acute coronary syndrome versus stable angina. Circ Cardiovasc Interv. 2012;5:55-61.
- Oemrawsingh RM, Garcia-Garcia HM, van Geuns RJ, Lenzen MJ, Simsek C, de Boer SP, Van Mieghem NM, Regar E, de Jaegere PP, Akkerhuis KM, Ligthart JM, Zijlstra F, Serruys PW, Boersma E. Integrated biomarker and imaging study 3 (IBIS-3) to assess the ability of rosuvastatin to decrease necrotic core in coronary arteries. EuroIntervention. 2016;12:734-9.
- Kini AS, Baber U, Kovacic JC, Limaye A, Ali ZA, Sweeny J, Maehara A, Mehran R, Dangas G, Mintz GS, Fuster V, Narula J, Sharma SK, Moreno PR. Changes in plaque lipid content after short-term intensive versus standard statin therapy: the YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy). J Am Coll Cardiol. 2013;62:21-9.
- Brugaletta S, Magro M, Simsek C, Heo JH, de Boer S, Ligthart J, Witberg K, Farooq V, van Geuns RJ, Schultz C, van Mieghem N, Regar E, Zijlstra F, Duckers HJ, de Jaegere P, Muller JE, van der Steen AF, Boersma E, Garcia-Garcia HM, Serruys PW. Plaque compositional Syntax score: combining angiography and lipid burden in coronary artery disease. JACC Cardiovasc Imaging. 2012;5:S119-21.
- Madder RD, Khan M, Husaini M, Chi M, Dionne S, VanOosterhout S, Borgman A, Collins JS, Jacoby M. Combined near-infrared spectroscopy and intravascular ultrasound imaging of pre-existing coronary artery stents: can near-infrared spectroscopy reliably detect neoatherosclerosis?. Circ Cardiovasc Imaging. 2016;9: e003576.