Lesion and patient subsets: chronic kidney disease (old)
Summary
Chronic kidney disease (CKD) is among the strongest predictors of adverse outcome in patients with coronary artery disease (CAD) undergoing myocardial revascularisation. Similarly, cardiovascular morbidity and especially CAD is the main cause of death in patients with CKD.
Even though CKD patients have an increased cardiovascular risk profile, they have lower rates of cardiac catheterisation and myocardial revascularisation and they are less likely to receive guidelines-directed medical therapy compared to patients with preserved renal function.
Management of CAD in patients with CKD requires careful evaluation of risks and benefits considering that any revascularisation implies the risk of worsening the renal function.
In patients with multi-vessel CAD and moderate CKD (stage 3 or lesser), surgery is preferred to percutaneous coronary intervention (PCI) when the patient’s risk profile is acceptable and life expectancy is reasonable due to the better survival and lower recurrence of ischaemia, in particular when diabetes is the cause of CKD. In patients with severe CKD (stage 4 to 5) or in haemodialysis, the revascularisation strategy must be set taking into account the general condition of the patient and his or her life expectancy, with the less invasive approach being more appropriate for the most fragile and compromised patients.
When percutaneous coronary intervention (PCI) is performed in patients with advanced CKD and diffuse or calcified CAD, accurate lesion preparation, including rotational atherectomy or coronary lithotripsy, is essential to obtain adequate stent expansion. Dedicated ultra-low/zero contrast volume PCI protocols may be considered in high-risk patients. The use of intracoronary imaging and physiology should be implemented to reduce the contrast volume and optimize PCI results.
Every effort should be made to prevent contrast-induced acute kidney injury (CI-AKI) after PCI to prevent worsening of the renal function. A number of specific techniques and devices have been developed to prevent CI-AKI, even though adequate pre and post-procedure hydration (especially if tailored on patient’s left ventricle end diastolic pressure or central venous pressure) and contrast volume minimization remain the most effective preventive measures.
Structural heart interventions and, in particular, TAVI, are often performed in patients with CKD. The presence of CKD is associated with worse clinical outcome after TAVI. However, kidney function recovery has been consistently observed after TAVI, unlike surgical valve replacement that needing extracorporeal circulation adds damage to the renal function. Therefore, TAVI may offer advantages compared to surgery in patients with CAD associated to severe aortic stenosis and CKD and should be considered even in high risk CKD patients to improve survival.
Introduction
Definition of CKD
Glomerular filtration rate (GFR) is accepted as the best measure of kidney function. Normal values are dependent on age, sex and body size and are approximately 100-130 ml/min/1.73m2 in young men, and 90-120 ml/min/1.73m2 in young women.
The National Kidney Foundation has classified patients with CKD in 5 different stages according to the progressive reduction of the estimated GFR (eGFR) and evidence of renal damage. A patient has CKD stage 1 when the eGFR is higher than 90 ml/min/1.73m2 but there is evidence of renal damage (i.e proteinuria). The second stage corresponds to a mild reduction of the eGFR to 60-89ml/min/1.73m2 with evidence of renal damage (mostly proteinuria at the standard urine examination). Stage 3 CKD corresponds to a moderate reduction of the eGFR to 30-59 ml/min/1.73m2 and the stage 4 corresponds to a severe reduction of the eGFR to <30ml/min/1.73m2. The stage 5 (or end-stage renal disease: ESRD) refers to patients in kidney failure, with eGFR<15 ml/min/1.73m2, requiring, or close to requiring, replacement therapy.
Measurement of the GFR
The direct measurement of the plasma clearance (Cl) of an ideal filtration marker, such as inulin, represents the gold standard for the determination of GFR in humans, but this is complex, expensive and not feasible in routine practice. Several formulae have been developed to estimate GFR rapidly using serum creatinine values, the most largely accepted and validated being the Cockcroft-Gault formula, and the calculation of the eGFR using the Modification of Diet in Renal Disease Study (MDRD) equation, .
Cockcroft-Gault formula: Cl Creatinine= [(140-age) x weight/](72xSC)x0.85 where ClCr is expressed in ml x min, age in years, weight in Kg, and SC in mg/dL.
MDRD 4
Revised equation: GFR= 175 x (standardized SC)-1.154x(age)–0.203 x0.742 (if the subject is female) or 1.212 (if the subject is black).
Accuracy of the two formulae are similar, the MDRD being more precise in older and obese people.
The use of calculated clearance to determine the renal function is encouraged instead of serum creatinine or serum cystatin-c values alone, in particular in the elderly.
Robust scientific evidence indicates a cut off value of 60ml/min/m2 GFR to establish the diagnosis of mild CKD. This value correlates significantly with the occurrence of major cardiovascular events, including mortalit. Furthermore, in diabetic patients, the diagnosis of proteinuria alone supports the diagnosis of CKD with similar prognostic implications due to diabetic macroangiopathy independently of the GFR.
The cardio-renal unit
The prevalence of CKD in Europe varies from 3.3% to 17.3% according to national registries and it is higher in the population segment aged 45-73 years where it ranges from 6.3% to 25.6%. In particular, the number of patients with end stage renal disease (ESRD) is progressively increasing.
Contemporary data show that kidney failure requiring renal replacement therapy (RRT) is present in a range between 5.3 milion and 9.7 milion people worldwide.
CKD prevalence increases with age, and, as the life expectancy continuous to grow, the prevalence of CKD increases among the general population. Moreover, patients with CKD have also an increased prevalence of other coronary artery disease (CAD) risk factors including diabetes. Therefore, the combination of CKD, diabetes and CAD is a frequent observation in patients treated in the catheterization laboratory.
Renal function is directly associated with survival and, in particular, CKD increases the risk of cardiovascular events and portends a worse prognosis for any of the clinical presentations of CAD.
In patients with acute myocardial infarction (MI) in-hospital mortality ranges from 2% among patients with normal renal function, to 6%, 14%, 21% and 30% in patients with mild, moderate, severe, or dialysis-requiring CKD, respectively; a trend that is also observed long-term after discharge, , .
Among patients with a non-ST-elevation acute coronary syndromes (NSTE-ACS), those with mild or moderate CKD have a significantly higher 30 day and 6 month mortality compared to patients with preserved renal function, .
In patients with chronic coronary syndrome or non-symptomatic CAD, the risk of MI or cardiac death in patients with mild-to-moderate and in patients with severe CKD is respectively 2.3-fold and 5.1-fold higher compared with patients with preserved eGFR.
Furthermore, patients with CKD and angiographically unobstructed coronary arteries have a higher rate of death (24.7% vs 3.9%, HR 6.5, 95%CI 3.1-13.7, p<0.001) or MI (5.2% vs 0.7%, HR 10.2, 95%CI 1.7-62.3, p=0.001) compared with patients with unobstructed coronary arteries but preserved renal function.
CKD accelerates atherosclerosis, myocardial disease, and valvular heart disease as well as promoting a favourable substrate for cardiac arrhythmias. This accelerated vascular pathobiology represents the most important reason why patients with CKD have a poor prognosis when CAD becomes symptomatic, .
CKD pathophysiology is associated with accelerated cardiovascular morbidity and mortality. Notably, CKD causes dyslipidaemia decreasing the function of lipoprotein lipase with consequent reduction of HDL-cholesterol, elevated triglycerides and normal LDL-cholesterol. Furthermore, CKD courses with hypocalcaemia, hypophosphatemia and hyperparathyroidism, a biological milieu that, along with metabolic acidosis causes bone decalcification and vascular calcification with a consequent acceleration of the atherosclerotic process. CKD is also known to be an inflammatory condition promoting plaque vulnerability and rupture leading to cardiovascular events. In addition, the chronic hyperactivation of the adrenergic system and the renin-angiotensin system worsens hypertension augmenting the intravascular wall stress that may further contribute to the development of acute vascular events. Anaemia due to erythropoietin deficiency may also contribute together with hypertension in determining left ventricular hypertrophy. Hyperkalaemia, and other fluid-electrolytic imbalances can promote atrial and ventricular arrhythmias that may contribute to cardiac mortality.
Patient care in earlier stages of chronic kidney disease (CKD) should be focused on prevention of cardiovascular complications. Patients with CKD require strict cardiovascular control and an active evaluation of revascularisation strategies, independently of the clinical presentation of the CAD. Furthermore, preventive measures to avoid worsening of GFR and optimisation of medical treatment are essential to improve the risk-benefit balance of any revascularisation strategy in this delicate sub-set of patients.
Coronary interventions in patients with CKD
Myocardial revascularization in patients with CKD
Myocardial revascularisation of patients with CKD, by either surgical or percutaneous means, imposes particular attention to some specific characteristics of these patients. Other than age, left ventricular function and the degree of vessel involvement, the presence of renal dysfunction is a strong determinant for the estimation of the postoperative clinical risk for patients undergoing coronary artery bypass grafting (CABG) as estimated by the EuroSCORE. Similarly, CKD is associated with diffuse CAD and more extensive calcification of the artery walls and these are important variables included in the evaluation of the procedural risk related to PCI in the SYNTAX score.
Long-term follow of contemporary randomized trials comparing PCI and CABG demonstrated substantial equivalence of percutaneous and surgical myocardial revascularization in terms of hard clinical outcomes in complex CAD scenarios up to 5 years of follow up, . Therefore, considering the incremental risk that CKD confers on surgical interventions, this should be reserved for patients with a good chance of long-term survival in order to derive sufficient benefit to justify the initially augmented risk.
In common practice, patients with CKD are less likely to receive invasive management of CAD compared with patients with preserved renal function both for acute coronary syndrome (OR 0.60, 95%CI 0.59-0.61) and for chronic coronary syndrome (OR 0.54, 95%CI 0.40-0.73).
The randomized International Study of Comparative Health Effectiveness With Medical and Invasive Approaches-Chronic Kidney Disease (ISCHEMIA-CKD) trial compared an initial invasive strategy (coronary angiography with PCI or CABG as clinically indicated) vs initial conservative management in patients with advanced CKD (eGFR <30 ml/min or RRT) and stable angina with evidence of moderate or severe myocardial ischemia. Notably the investigators found that an initial invasive strategy did not result in a lower incidence of all-cause mortality or MI (HR 1.01, 95%CI 0.79-1.29, p=0.95) at a median follow up time of 2.2 years (interquartile range 1.6-3.0 years). Conversely, the initial invasive strategy was associated with increased incidence of stroke (HR 3.76, 95%CI 1.52-9.32, p=0.004) and of the safety outcome (composite of death from any cause and initiation of dialysis; HR 1.48, 95%CI 1.04-2.11, p=0.03).
These data should help us revising the indications for treatment in patients with advanced CKD and stable CAD presentation. However, in the interpretation of the ISCHEMIA-CKD findings, it must be considered that patients who were very symptomatic, had recent ACS or who had heart failure or LVEF <35% were excluded from the trial.
Complete revascularization
Complete revascularization should remain the main goal of treatment for patients with CKD and CAD. Indeed, complete revascularization improves the outcome of patients with CKD as much as it does in patients with preserved renal function .
Data from a large PCI Registry from Korea recently showed that, in patients with CKD undergoing PCI with second generation DES implantation, complete revascularization (residual SYNTAX score=0) reduces the risk of death, myocardial infarction and any revascularization compared with incomplete revascularization (residual SYNTAX score >0) (HR: 0.79, 95% CI 0.64-0.96, p=0.020). Importantly, the favourable outcome was driven mainly by less need for new revascularization at 3 years of follow up. Furthermore, no data on the impact of extensive revascularization on the residual renal function was available for these studies.
This novel data is in contrast with previous observations in CKD patients treated with first-generation DES implantation, in whom angiographic complete revascularization was associated with higher risk of all-cause mortality and MI in patients with CKD.
Multivessel disease
CKD is associated with diffuse and accelerated atherosclerosis, hence multivessel disease (MVD) is common in this setting. In patients with MVD, CABG should be preferred over PCI when the extent of the CAD is significant, when the patient’s risk profile is acceptable and when life expectancy is reasonable.
In fact, there is consistent evidence supporting CABG as a better treatment than PCI in patients with moderate and severe CKD and multi-vessel CAD, in terms of survival and recurrence of ischaemia, in particular when diabetes is the cause of the CKD, , , .
The results of the 5-year outcome of the SYNTAX trial demonstrated that differences between PCI and CABG in rates of adverse events were larger in patients with CKD than in patients with preserved renal function. Notably, in patients with CKD, the rate of MACCE was significantly higher in patients who underwent PCI compared with those who underwent CABG (42.1% vs 31.5%, p=0.019), especially in patients with diabetes and extensive CAD.
The recently published 10-year outcome of the SYNTAX trial did not show significant difference in all-cause mortality between PCI and CABG (HR 1.19, 95%CI 0.99-1.43, p=0.066). Surprisingly, there was no significant difference in survival even in the subgroup of patients with diabetes (p for interaction=0.60). However, importantly, no prespecified subgroup analysis was performed in patients with CKD.
A recent metanalysis by Wu P et al. including 62,343 patients with CKD and multivessel CAD, did not show significant difference in MACCE (OR 1.58, 95%CI 0.99-2.52) or overall mortality (OR 1.08, 95%CI 0.95-1.23) between PCI and CABG. PCI was associated with higher risk of cardiac mortality (OR 1,29, 95%CI 1.21-1.37), myocardial infarction (OR 1.73, 95%CI 1.35-2.21), repeat revascularization (OR 3.9, 95%CI 2.99-5.09) but lower risk of short-term mortality (OR 0.56, 95%CI 0.37-0.84) and cerebrovascular events (OR 0.65, 95%CI 0.53-0.79) compared with CABG.
In patients with end-stage renal disease or requiring RRT CABG has shown better survival and lower rate of reinterventions at long-term follow-up compared with PCI, , , . Nevertheless, in this subgroup, PCI was associated with lower risk of post-procedural mortality (1.0% vs 1.7%; HR= 0.55; 95%CI:0.35-0.87; p=0.01) and lower risk of peri-procedural stroke (0.4% vs 1.7%; HR= 0.22; 95%CI:0.12-0.42; p<0.0001) compared with CABG.
When surgical revascularisation is indicated in these patients, the off-pump technique may be preferred to conventional on-pump cardiac surgery. Although in general there is no consensus on the clear advantages of off-pump versus on-pump surgery, differences in favour of off-pump appear to be restricted to high-risk subgroups such as, for instance, patients with CKD. Furthermore, the advantages of off-pump surgery are more evident when it is performed routinely by trained and dedicated teams that have very low conversion rates to standard on-pump surgery and when the technique is applied to most patients, including those at low risk.
Left main
In the EXCEL trial patients with left main disease and CKD had increased incidence of the composite of mortality, stroke or MI compared with patients without CKD (HR 1.60, 95%CI 1.22-2.09, p=0.0005), mainly driven by a higher incidence of all-cause and cardiac mortality. At 3 years of follow up of the CKD subgroup, there was no difference in the composite of death, stroke and MI in patients undergoing left main PCI or CABG (HR 1.25, 95%CI 0.79-1.98, p=ns), similarly to what was observed for patients without CKD (p for interaction=0.36). Notably, PCI was associated with significant lower risk of acute renal failure compared with CABG (HR 0.28, 95%CI 0.09-0.87, p<0.05). Acute renal failure significantly increased the risk of adverse events at 3 years of follow up (HR 4.59, 95%CI 2.73-7.73, p<0.0001).
Conversely, the 5-year outcome analysis of the SYNTAX trial showed that CABG was associated with better outcome compared with PCI in patients with CKD and three-vessel disease and/or left main, especially when diabetes was also present. Notably, the 10-year analysis of clinical outcomes in the whole SYNTAX trial cohort showed that CABG did not provide survival benefit in patients with left main disease, whereas it did for patients with three-vessel CAD (p for interaction=0.023). Nevertheless, data on the CKD subgroup at 10 years were not presented.
A recent analysis of the IRIS-MAIN registry form Korea reported the clinical outcome at 2 years for patients with LMS CAD graded based on the renal function. Notably, patients with preserved renal function or mild-to-moderate CKD (eGFR ≥30 ml/min/1.73m) who underwent CABG or PCI presented similar risk of cumulative major adverse cardiovascular events. Conversely, in patients with severe CKD (eGFR<30 ml/min/1.73m), PCI was associated with significantly higher risk of MACCE (HR 1.88, 95%CI 1.08-3.25, p=0.02) compared with CABG.
Further adequately powered trials are needed to establish the optimal revascularization strategy in patients with CKD and evidence of LMS CAD.
Anaemia and bleeding risk in patients with CKD
Anaemia is a constant feature in patients with CKD and is associated with volume overload, diastolic dysfunction, respiratory changes due to acidosis and physical deconditioning. Anaemia significantly contributes to worsen secondary ischaemia. The correction of anaemia with chronic administration of erythropoietin is recommended, with a target Hb range of 11.0-12.0 g/dL, to ensure the adequate long-term management of patients with CKD.
Anaemia secondary to bleeding after PCI is also an important independent predictor of adverse outcome. Patients who experienced periprocedural bleeding have a higher likelihood of developing acute kidney injury, and its extent correlates closely with bleeding severity. Perioperative blood loss should thus be minimised, especially in patients at a priori high risk of acute kidney injury. In these patients performing PCI via a trans-radial approach is preferable, because the risk of bleeding is significantly lower compared with the trans-femoral approach, , , . Transfemoral approach has been associated with a higher incidence of renal injury after percutaneous procedures compared with trans-radial approach, , although this finding has not been confirmed by all studies.
The overall annual risk for bleeding is significantly higher in patients with CKD compared with the general population (approximately 2.5% per year compared with <1%), and it increases with decreasing renal function. Moreover, impaired renal clearance of many pharmacological agents increases the risk of overdosing, augmenting this higher bleeding risk further.
In this particular setting the antiplatelet therapies’ risk-benefit ratio may be altered since the absolute bleeding risks with antiplatelet therapy might be at least doubled in patients with CKD.
The best antiplatelet strategy in patients with CKD is currently not known. Antiplatelet agents reduce myocardial infarction in CKD patients but have uncertain effects on stroke and mortality and may increase bleeding.
In the Clopidogrel for Reduction of Events During Obstervation (CREDO) trial, Clopidogrel failed to demonstrate superiority over placebo in CKD patients, showing rather a trend in the opposite direction, with an absolute increased event rate in patients with renal impairment.
A recent sub-analysis of the PEGASUS-TIMI 54 trial evaluated the efficacy and safety of
Ticagrelor in stable patients with prior myocardial infarction and impaired renal function (eGFR<60ml/min/1.73m2). Ticagrelor reduced the risk of major events similarly in patients with or without renal failure (HR 0.81, 95%CI:0.68-0.96 vs HR:0.88, 95%CI:0.77-1; p for interaction=0.44). In patients with a history of infarction, however, renal dysfunction increased the risk of ischaemic events (HR:1.54; 95%CI 1.27-1.85; p<0.0001) and therefore, the absolute risk reduction with Ticagrelor was greater for CKD patients (2.7%, 95%CI 0.49-4.93 vs 0.63%, 95%CI 0.32-1.57). At the same time bleeding occurred more frequently in CKD patients, but the incidence of TIMI major bleeding was similar in the two groups (HR 1.19; 95%CI 0.64-2.24; p=0.58).
Importantly, potent P2Y12-ADP antagonists are expected to provide benefit in CKD presenting with acute coronary syndromes because these patients exhibit an overall increased ischemic risk and a reduced efficacy of Clopidogrel. Even though there are no dedicated randomized studies in patients with CKD, the clinical effect of Ticagrelor and Prasugrel in this setting can be evaluated by subgroup analysis of recent trials and registries.
A sub-study of the Platelet Inhibition and Patient Outcomes (PLATO) trial demonstrated that Ticagrelor, compared with Clopidogrel, reduced mortality and myocardial infarction in acute coronary syndromes patients and impaired renal function, with no significant increase in major or fatal bleeding. Of note in this study the number of major bleeding events increased with decreasing of creatinine clearance and was similar in the Ticagrelor and Clopidogrel groups.
Results from the SWEDEHEART Registry showed that Ticagrelor provided lower rate of death, MI and stroke at 1-year follow-up compared with Clopidogrel (HR, 0.82; 95% CI, 0.7–0.97) in patients with moderate CKD. Conversely, no benefit was observed for patients with severe CKD (adjusted HR, 0.95; 95% CI, 0.69–1.29). Moreover, patients with severe CKD treated with Ticagrelor presented a trend towards increased risk of bleeding (HR, 1.79; 95% CI, 1.00–3.21).
In the TRITON TIMI 38 trial, patients with acute coronary syndrome were randomized to Prasugrel or Clopidogrel. Notably, the benefit of Prasugrel over Clopidogrel was maintained in the CKD subgroup (p for interaction=ns). However, the study did not report data on the bleeding risk in this subgroup.
In the large PROMETHEUS registry Prasugrel was not superior to Clopidogrel in patients with CKD and acute coronary syndrome in reducing the composite endpoint of death, MI, stroke and urgent revascularization (HR 1.0, 95%CI 0.8-1.25)
Conversely, in a recent combined analysis of the RENEMI and BLEEMACS registries, potent P2Y12 inhibitors significantly reduced the mortality rate (HR 0.82, 95% CI 0.54-0.96; p= 0.006) and the risk of reinfarction (HR 0.53, 95% CI 0.30-0.95; p= 0.033) in CKD patients as compared to clopidogrel.
In conclusion, overall, potent P2Y12-ADP antagonists presented a positive risk benefit ratio in patients with CKD. In patients with moderate CKD Ticagrelor provides benefit over Clopidogrel, whereas there is still uncertainty on the benefit of Prasugrel in this setting. Importantly, data on patients with severe CKD are scarce and evidence is lacking to support the use of potent P2Y12-ADP antagonists in this setting.
Calcified CAD in patients with CKD
Diffuse disease and severe calcification are common features of CAD in patients with CKD.
As discussed previously, the pathobiology of the arterial vessel wall in patients with CKD favours calcification. In fact, post-mortem studies have demonstrated increased media thickness, smaller lumen area, and much higher calcium scores in CKD patients compared with age and gender matched control subjects. Diffuse disease and coronary calcifications constitute important prognostic variables in patients undergoing PCI, and this is expressed in the SYNTAX score algorithm.
Extensive coronary calcifications often require some dedicated interventions in order to achieve acceptable results. In particular, rotational atherectomy or coronary lithotripsy therapy should be considered for the treatment of diffusely calcified vessels and non-dilatable stenosis.
Intravascular ultrasound may be considered to guide diagnosis and interventions in complex CAD scenarios in patients with CKD to optimise treatment in calcified vessels (Figure 1). Optimal stent expansion should be a main objective in order to reduce both, the risk of stent thrombosis and the risk of restenosis, which are more frequent in CKD patients compared with general population, .
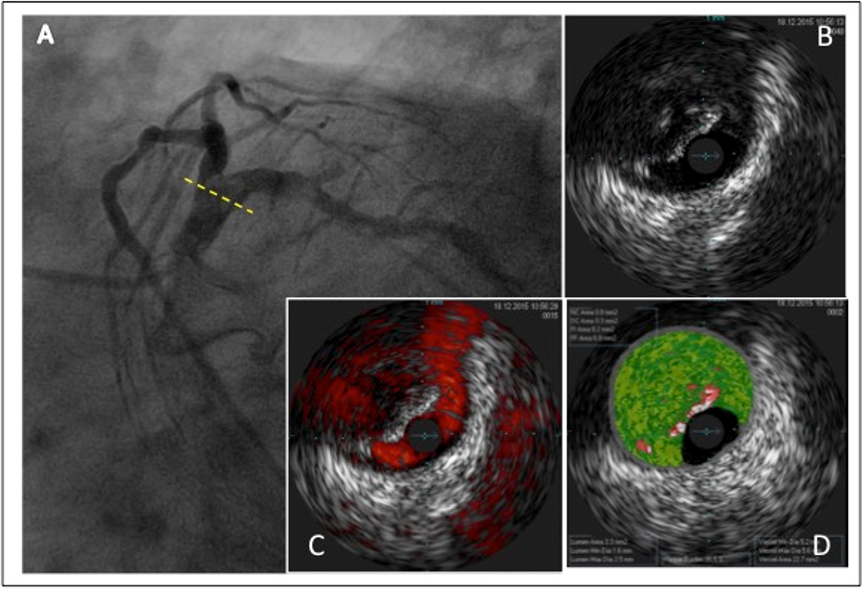
Figure 1
A patient with CKD presented with unstable angina and underwent coronary angiography. This showed evidence of a focal hypodense lesion in the distal left main coronary artery causing an angiographically non-significant stenosis (A). Intravascular ultrasound including virtual histology, however, showed severe calcified lesions in the left main coronary artery (B, C, D), left anterior descending and circumflex coronary artery. The patient was referred for and underwent coronary artery bypass graft surgery.
Coronary physiology in patients with CKD
The accuracy of non-invasive tests for the functional assessment of myocardial ischemia is limited by the high rate of false positive and false negative tests in patients with CKD. Exercise testing and pharmacological perfusion imaging may have reduced accuracy for detecting CAD in CKD, given the high pre-test probability of atherosclerosis and the moderate at best sensitivity of non-invasive tests. Therefore, invasive coronary physiology may play an important role in guiding myocardial revascularization in patients with CKD, detecting ischemia-provoking lesions and potentially avoiding unnecessary PCI (Figure 2, Figure 3). However, evidence on the reliability of physiological indices in patients with CKD is scarce and CKD patients are underrepresented in most of the landmark coronary physiology studies. CKD was present in only 2.2% of FAME 2 trial cohort, and CKD rate was not reported for DEFER, DEFINE-FLAIR and iFR-SWEDEHEART trials.
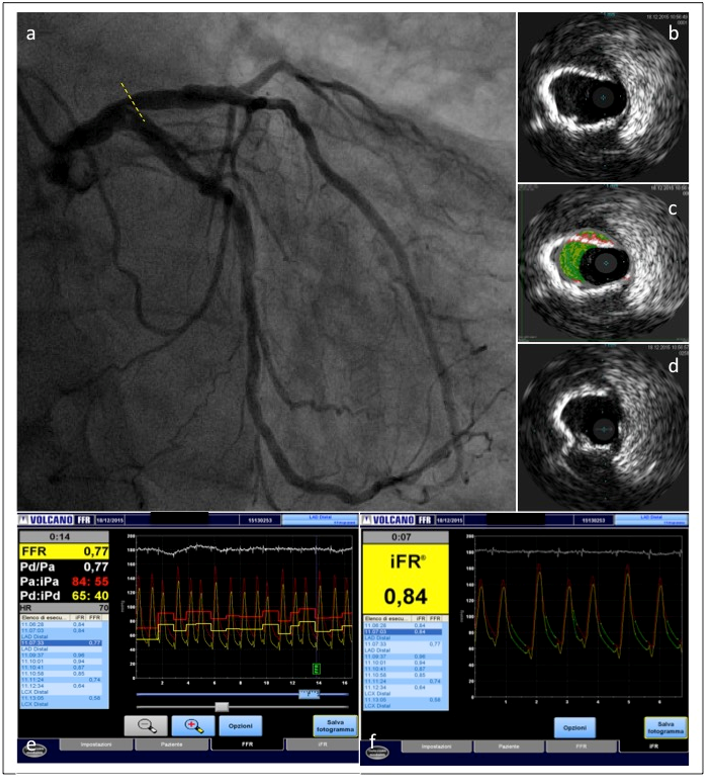
Figure 2
Coronary angiography in the same CKD patient showing a lesion located in the proximal left anterior descending coronary artery (a). The lesion was studied with IVUS and functionally assessed with instantaneous wave-free ratio (iFR) and fractional flow reserve (FFR) measurement. IVUS showed a severe calcified lesion (b,c,d). The severity of the coronary stenosis was confirmed by iFR-FFR measurements(e,f).
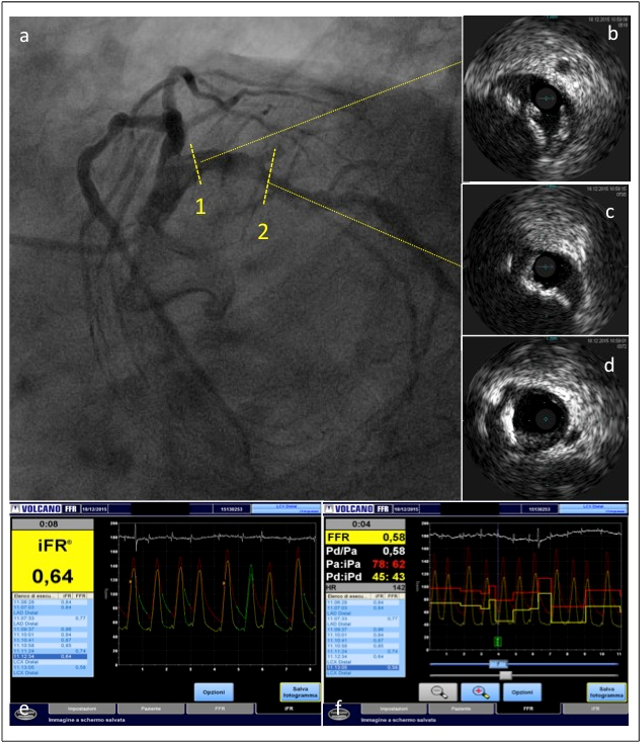
Figure 3
Left circumflex coronary artery of the same patient. IVUS showed a severe calcified lesion. The severity of the coronary stenosis was confirmed by both FFR and iFR measurements.
CKD is associated with impaired microvascular function and, thus, the vasodilatory response to any hyperaemic agent may be impaired in this setting. In the FREAK study, the investigators observed a larger proportion of negative FFR in patients with impaired renal function. Notably, there was an association between the degree of renal dysfunction and the value of FFR. In particular, the more severe the renal dysfunction the more often the FFR was negative, suggesting a possible underestimation of the true ischemic potential of a given coronary lesion. The same authors demonstrated that the index of microcirculatory resistance (IMR), a specific index of coronary microvascular function, was significantly higher in patients with CKD, confirming the possible suboptimal hyperaemia achievement in this setting.
Other investigators reported a suboptimal improvement of coronary physiology after PCI in patients with CKD. This fact may be related to the diffuse coronary disease and the degree of coronary calcifications in patients with CKD. In presence of a heavily calcified coronary artery in fact, the geometry of calcifications might interact with coronary blood flow and might be associated with a loss of flow energy accounting for the blunted hyperaemic response to adenosine and the poor correlation between physiology and angiographic severity. Being independent from vasodilatory response, iFR could potentially show an advantage over FFR in CKD patients. However, no specific data on the accuracy of iFR in CKD is currently available.
Dedicated studies are needed to elucidate the benefit of physiology-guided revascularization in patients with CKD. However, from a practical point of view, it can be assumed that physiology indices are reliable in guiding revascularization when below the ischemic threshold. Conversely, careful FFR/iFR values interpretation is recommended in case of a negative or borderline result, especially in patients with advanced CKD and heavily calcified coronary arteries.
Contrast-induced acute kidney injury
Contrast-induced acute kidney injury (CI-AKI) is defined as an acute impairment of the renal function after iodinated contrast medium administration in the absence of another cause, .
CI-AKI is expressed according to different definitions as a relative increase in serum creatinine concentration within 48-72 hours of contrast exposure of either at least 25%, or as an absolute increase of 0.5mg/dl or 0.3mg/dl, . The renal injury of CI-AKI is normally transient with return to basal function within approximately one week following exposure to iodinated contrast medium. Nevertheless, CI-AKI may lead to irreversible renal damage and it is associated with the clinical outcome in patients undergoing PCI, including increased hospital stay, need for dialysis and all-cause mortality, .
The pathophysiology of CI-AKI is multifactorial, and it is associated with direct and indirect effect of the contrast medium and with periprocedural ischemic damage caused by renal hypoperfusion and embolization. Contrast dye may have a direct nephrotoxic effect on the tubular epithelium leading to tubular cells apoptosis and necrosis. On the other hand, contrast may exert an indirect effect, causing an exaggerated vasoconstriction in the afferent and efferent glomerular arterioles leading to ischemic damage.
Recent data reported an incidence of CI-AKI of approximately 7% in all-comers population. However, the incidence of CI-AKI may increase up to 25% in high risk subsets of patients, .
Pre-existing CKD and the volume of contrast medium injected are the most important predictors for CI-AKI. Among other patient-related factors, older age, female sex, diabetes, anaemia and heart failure with hypovolaemia or low cardiac output have been shown to correlate with the risk of CI-AKI, .
Estimation of the risk for developing or worsening renal insufficiency
All patients scheduled for PCI should be correctly informed about the potential benefits and risks of the procedure, including the risk of a procedure-related worsening of the renal function. Patients with known CKD are exposed to a particularly higher risk of suffering further impairment of the renal function, and eventually the need for permanent dialysis, despite the conscious implementation of preventive measures.
Several risk scores to predict CI-AKI after PCI have been developed, , . The CIN risk score recently validated in a large multicentre population of patients who underwent PCI comprises of pre-existing renal disease, use of metformin, history of previous PCI, peripheral arterial disease and >300ml contrast volume administered during the procedure. If the total score is ≥3, then the patient is considered to be at high risk of CIN (>20%).
Another validated score takes into account several known risk factors for renal injury after PCI: age >75 years, diabetes mellitus, congestive heart failure, hypotension, anaemia, the use of intra-aortic balloon pump, the volume of contrast media and the presence of a reduced GFR (<60ml/min/1.73m2) or augmented serum creatinine (>1.5mg/dl). The risk of CI-AKI varies significantly through the categories at low (7.5%), moderate (14%), high (26%) or very high risk (57.3%). Of note, this risk score also predicts one-year mortality.
CI-AKI preventive measures
There is no specific treatment for CI-AKI. Therefore, adequate prevention is paramount.
Strategies to prevent CI-AKI focus on the following mechanisms: 1) intravascular volume expansion; 2) minimise contrast volume; 3) avoid periprocedural renal hypoperfusion; 4) optimise medical therapy.
-
Intravascular volume expansion
Intravascular volume expansion is the most important preventive measure for CI-AKI.
All patients with CKD undergoing a diagnostic catheterisation or PCI should receive preventive hydration with isotonic saline started at least 12 hours before angiography and continued at least 24 hours afterwards to reduce the risk of CI-AKI.
Infusion rates should be standardized at 1ml/kg/h of 0.9% saline, except in cases of severe left ventricular dysfunction (ejection fraction <35%) when the infusion rate should be reduced to 0.5ml/kg/h. Furosemide with matched hydration may be considered over standard hydration in patients at very high risk of CI-AKI and in patients with advances heart failure. (Class IIb A)
Recently, intravascular volume expansion strategies tailored on left ventricle end-diastolic pressure (LVEDP), central venous pressure (CVP) or based matched-hydration systems have demonstrated encouraging results.
The Prevention of Contrast Renal Injury with Different Hydratation strategies (POSEIDON) Trial demonstrated that the LVEDP-guided strategy significantly reduced the risk of CI-AKI compared with standard hydration (RR: 0.41, 95%CI 0.22-0.79, p=0.005).
Similarly, CVP-guided strategy demonstrated advantages in reducing the risk of CI-AKI over standard hydration in a cohort of patients with CKD and heart failure (15.9% vs 29.5%, p=0.006)
Finally, the RenalGuard system[TM] (PLC Medical Systems, Inc, Franklin, MA, USA) is a fluid management device projected to achieve high urine output and contemporarily minimise the risk of volume depletion by using a high-volume fluid pump to infuse saline and replete volume. The infusion rate is adjusted second by second in response to changes in urine output in order to avoid volume depletion or overload . In the Renal insufficiency After Contrast Media Administration (REMEDIAL II) trial, the use of RenalGuard was associated with a significant reduction in the risk of CI-AKI (11% vs 20%, OR: 0.47, 95% CI 0.24-0.92) compared with standard of care in high risk CKD patients.
-
Contrast volume minimization
Contrast-volume-to-creatinine-clearance (CV/CrCl) ratio > 2 is associated with significant increased risk of CI-AKI in patients with advanced CKD (eGFR <30 ml/min/1.73 m). In this setting the operator should aim for CV/CrCl <1 to minimize the risk for CI-AKI.
In complex procedures and vulnerable patients diluted contrast with 50% of normal saline may be an acceptable strategy to reduce CV/CrCl and obtain still acceptable image quality. The procedural steps for ultra-low/zero contrast volume PCI procedures are described in detail below.
In selected high-risk procedures, the use of a dedicated device such as the DyeVert PLUS System (Osprey Medical, Minneapolis, Minnesota) may be considered.
DyeVert PLUS is connected between the injection syringe and the manifold via a 4-way stopcock, allowing diversion of excess contrast during manual injection. In a recent randomised study, the use of DyeVert PLUS was associated with a relative reduction of 15% of contrast volume during coronary angiography and PCI but similar rates of CI-AKI compared with the control group.
Coronary sinus aspiration immediately after contrast injection is an alternative strategy to reduce the contrast volume. In a small non-randomized study CI-AKI rate was lower in the coronary sinus aspiration compared with controls (5.5% vs 36.0%, p=0.03).
-
Avoid renal hypoperfusion
Renal hypoperfusion is an important determinant of CI-AKI. Any effort should be attempted to reduce the risk of periprocedural hypotension especially in high risk patients.
The choice of the vascular access is thus important. In fact, radial access is associated with reduced risk of bleeding and renal hypoperfusion and should be preferred whenever possible.
Moreover, femoral access may be associated with the risk of renal embolization, particularly in patients with atherosclerotic aortic disease or with evidence of peripheral artery disease or diffuse multilevel atherosclerosis. Renal-embolic complications are unpredictable, independent from the quantity of contrast media and do not correlate with the basal renal function. Unlike CI-AKI the impairment of renal function is accidental, progressive and often permanent; it ensues slowly several days after catheterisation and is often diagnosed late after discharge. The real incidence of this severe complication is debated and ranges between about 1.5% in unselected populations undergoing cardiac catheterisation and 8% in cases with severe atherosclerosis undergoing PCI, .
Post-PCI monitoring of the renal function.
Early indicators of CI-AKI could allow an early diagnosis and therefore reduce treatment delay. At the same time, markers with good negative predictive value could safely support early discharge protocols for those unlikely to develop CI-AKI. On this specific issue there is some debate on which is the best early predictor of renal injury after angiography and interventions. Cystatin-c is a cationic low-molecular weight cysteine protease produced by all nucleated cells at a constant rate, it is not metabolised in the serum, and is freely filtered by the glomeruli; therefore, it was proposed as an alternative to serum creatinine to evaluate the GFR without variations related to age, gender and muscle mass, . Furthermore, changes of serum cystatin-c have been shown, in some centres experiences, to be superior to serum creatinine in detecting early changes of GFR after administration of contrast media, . However, cystatin-c did not demonstrate prognostic advantages compared to serum creatinine, .
Therefore, in the clinical practice, serum creatinine remains the standard method for monitoring the renal function after angiography since a diagnostic superiority of serum cystatin-c over creatinine has not been established. It has been recently demonstrated that percent changes of serum creatinine detected as early as 12 hours after exposure to contrast media are much more informative than absolutes variations. The use of a delta of serum creatinine instead of absolute values to determine variations of renal function obviates for the most part limitations of creatinine and highly enhances its diagnostic sensitivity. Indeed, a +5 to +10 percentage change as early as 12 hours from baseline corresponds to creatinine elevations that would be considered negligible in clinical practice. However, such changes predict very early the occurrence of CIN with reasonably high sensitivity and specificity without adding additional cost or time to routine practice, .
Ultra-low contrast volume PCI
In high-risk CKD patients, an ultra-low contrast volume strategy may be considered to optimize the outcome of PCI. The following zero-contrast-PCI protocol has been proposed by Ali and colleagues in a small cohort of 31 high risk patients (average eGFR 16 ml/min/1.73 m). Notably, the protocol allowed to successfully perform PCI in all the cases without significant worsening of the renal function.
Here below are reported the procedural steps and point of discussion adapted from Ali et al. experience.
- Initial diagnostic angiogram should aim to minimize the contrast volume, ideally maintaining a CV/CrCl ratio <1 in high risk patients (eGFR <30 ml/min/1.73 m). Staged PCI should be considered after initial diagnostic angiogram, in order to allow renal function recovery, avoid contrast volume overload and allow appropriate informed consent. In general, ad-hoc PCI implies a larger dose of contrast media administration because of the addition of contrast agent during PCI to the amount of contrast already given for the diagnostic examination. It seems reasonable therefore to defer interventions after diagnostic angiograms in patients with impaired eGFR in particular when CAD presents angiographic characteristics that may require technically challenging interventions. Since differing interventions may sometimes be uncomfortable for the patients and unpractical for the institutions in some cases “ad hoc PCI” may be considered. This could be the case in simple, straightforward cases in which a minimum addition of contrast media is sufficient to perform PCI after angiography and no discussion with the heart team in required. Clear regulations for indications to perform ad hoc PCI have been reported by the ESC/EACTS.
- During staged PCI, reference images from the diagnostic angiogram should be uploaded on a side-screen in the cathlab to avoid unnecessary repeated contrast administration.
- Additional guidewires may be placed in side branches of the coronary artery to provide landmarks and guide PCI.
- Intracoronary imaging and, in particular, intravascular ultrasound (IVUS), is essential in ultra-low/zero contrast PCI protocols. In particular IVUS has an essential role in the preprocedural planning, identifying the proximal and distal landing zone and guiding the choice of stent length and size. Moreover, IVUS should be repeated following stent implantation to assess the result and check for stent under-expansion. Indeed, intracoronary imaging plays an important role in patients with severe CKD not yet on renal replacement therapy who need to undergo PCI. The Minimizing cOntrast utilization With IVUS Guidance in coronary angioplasty (MOZART) trial randomized patients with CKD to IVUS-guided vs angiography-guided PCI demonstrating a significant reduction in the CV/CrCl ratio (0.4[0.2-0.6] vs 1[0.6-1.9], p<0.001), but no significant difference in the occurrence of CI-AKI. Optical coherence tomography (OCT) may be considered in severe CKD patients using alternative substances for blood replacement than contrast dye such as dextran-40.
- Coronary physiology (FFR or iFR) should guide the indication for treatment. Reassessment of FFR/iFR at completion of PCI allow to evaluate the functional result of stenting without any further administration of contrast dye.
Transcatheter aortic valve implantation in patients with CKD
Patients with severe aortic stenosis who are candidate to transcatheter aortic valve implantation (TAVI) usually present high prevalence of baseline CKD , raising concerns about possible implication of procedure-related renal injury on outcomes.
The high incidence of CKD among these patients is due to the advanced age and the bidirectional relationship between AS and chronic renal damage. From one hand, renal disfunction accelerates aortic stenosis progression and increases its prevalence, by affecting bone and mineral metabolism and resulting in increased calcification of the aortic valve , . On the other hand, aortic stenosis by decreasing the cardiac output and increasing systemic venous congestion, reduces kidney perfusion and possibly accelerates or worsens the progression of CKD.
Given the progressive expansion of the indications of TAVI, the number of CKD patients undergoing percutaneous valve implantation is expected to progressively increase in the next years.
Therefore, it is paramount for clinicians and operators to better understand the clinical and procedural risk of bystander kidney dysfunction in patients undergoing TAVI, to improve its management and to investigate its impact on the outcomes.
When balancing the risk of kidney dysfunction progression associated with aortic valve replacement, it is important to understand that both surgical and transcatheter procedures are associated to an increased risk of acute kidney injury. Peri-operative hypotension, bleeding and extra-corporeal circulation , are known risk factors of SAVR-induced renal injury; similarly, bleeding, blood transfusion and contrast dye administration are associated with TAVI-related renal dysfunction, .
In aortic cardiac surgery, baseline CKD has been directly associated with increased early and late mortality, especially in patients with end-stage CKD . Moreover, historically, a high rate of patients did not receive SAVR based on their baseline kidney function .
There are no randomized data available to directly compare patients with severe CKD undergoing TAVI vs SAVR. In fact, patients with advanced CKD were excluded from most of the randomized clinical trials , .
Nevertheless, data from a large observational study focused on CKD patients undergoing TAVI vs SAVR, observed that patients undergoing TAVI present overall lower rate of AKI (OR 0.18, 95% CI 0.14 to 0.22, p < 0.001), AKI requiring dialysis (OR 0.30, 95% CI 0.20 to 0.44, p < 0.001), in-hospital mortality (OR 0.47, 95% CI 0.32 to 0.69, p < 0.001) and postoperative stroke (OR 0.27, 95% CI 0.13 to 0.53, p < 0.001) compared with patients undergoing SAVR.
CKD and overall mortality in patients undergoing TAVI
In patients undergoing TAVI, pre-existing CKD is associated with increased overall mortality (OR 2.532, 95% CI 1.01 to 6.35, p=0.048) . However, it remains unclear if mild CKD is associated with adverse outcome , , , .
Gargiulo et Al. conducted a meta-analysis including nearly 5000 patients aiming to assess impact of baseline kidney disease on TAVI outcomes. Notably, they observed that preoperative CKD, including stage 3, significantly increased early (OR 1.44; 95% CI, 1.08 to 1.94, p=0.01) and one-year (OR 1.66; 95% CI, 1.23 to 2.25; p=0.001) all-cause mortality . The same result emerged when the analysis was limited to patients with stage 3 CKD, confirming the impact of moderate CKD on TAVI outcome.
Accordingly, a study by Ferro et Al. confirmed the impact of kidney disfunction on mortality after TAVI and found a linear association between CKD severity and outcomes: for every 10ml/min/1.73m2 decrease in eGFR, in-hospital mortality increased by more than 8% and cumulative mortality at a medium follow-up of 18 months increased by over the 4%.
On top , the concomitant presence of diabetes (mortality at 30 days: OR 2.45; 95% CI, 1.19 to 5.07; p=0.02) or procedural features as trans-apical approach (mortality at 30 days: OR 3.12, 95% CI, 1.43 to 6.82; p=0.004), could worsen the impact of CKD on TAVI outcome.
CKD and procedural complications in patients undergoing TAVI
In patients undergoing TAVI, CKD impacts not only on mortality, but even on other complications risk. A significant correlation between baseline CKD (stage ≥4) and major/life-threatening bleeding (OR: 2.15, 95% CI 1.49 to 3.12, p = 0.001) was found in a multicentric study involving 2075 patients .
The same authors observed that the severity of baseline CKD was significantly associated with CI-AKI occurrence and need for dialysis post TAVI (P =0.001 for both).
However, a recent study by Adamo et Al. involving 2,733 TAVI patients from the Italian Clinical Service Project, found that severe CKD did not significantly modify the impact of postprocedural AKI in predicting early (p for interaction = 0.129) and 1 year mortality after TAVI (p for interaction = 0.386).
Regarding the need for dialysis, an analysis of the STS/ACC TVT registry (Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy) confirmed the correlation between baseline CKD and RRT. According to their data, more than 14% of patients with CKD stage 4 and nearly two third of patients with stage 5 required dialysis at one year after TAVI.
The higher risk for dialysis among patients with severe CKD should lead to cautious considerations within the Heart Team when planning the best management of severe aortic stenosis patients, including the possibility of a permanent renal replacement therapy following the intervention.
However, Steinmetz et Al. compared patients with stage 3-5 CKD and severe aortic stenosis undergone to TAVI or conservative treatment, based on clinician’s choice according to baseline characteristics including the deteriorated renal function. At 1 year, there was a significant decrease in renal function in the conservative group (39.6±13.9 ml/min to 34.4±15.3 ml/min) while in patients who underwent TAVI renal function remained unchanged (41.7±13 ml/min to 42.9±14.5 ml/min) (p-value=0.001).
The phenomenon of renal recovery after TAVI has been observed to be more significant in patients with baseline severely impaired renal function.
Voigtländer et Al., in a retrospective study including 540 patients, found a linear relation between pre-intervention renal function and post-intervention renal recovery, with patients with CKD class 4 and 5 having the larger increase in eGFR (19.54 ml/min pre TAVI vs. 27.9 ml/min after TAVI; p < 0.0001). Notably, in the same study, patients who had an improvement in eGFR after TAVI by more than 22% demonstrated an improvement in overall survival as well (p = 0.0068).
However, the real impact of post-TAVI kidney recovery on clinical outcomes is still under debate. In an analysis of PARTNER 1 trial, patients with improved eGFR at 30 days compared to patients without change in eGFR did not experience significant differences for all-cause mortality (15.4% vs 19.1%; p = 0.22), need for repeat hospitalization (12.3% vs 15.2%; p= 0.35), or any other clinical end points at 1 year follow up .
In conclusion, available data suggest TAVI may be a valuable therapeutic option for patients with CKD and concomitant aortic stenosis , . However, a case-by-case Heart team discussion must be performed especially for patients with advanced CKD, taking into considerations procedural strategies to prevent the risk of acute kidney injury and informing the patient about the risk of further renal function worsening and the possibility of need for renal replacement therapy (RRT).
CI-AKI and TAVI
Contrast-induced acute kidney injury (CI-AKI) is a common complication among patients undergoing TAVI.
The pathogenesis of CI-AKI is based on the toxic effect of contrast media. In addition, TAVI procedure is prone to a high risk of embolization of cholesterol debris to the renal vasculature, a phenomenon which, in current practice, is difficult to distinguish from CI-AKI.
Recently, it has been suggested that contrast dye plays only a limited role in the development of TAVI-related AKI, whereas other factors (i.e. bleeding, congestion, rapid pacing, hypotension, blood transfusions) are more strongly associated with TAVI-induced AKI.,
The incidence of CI-AKI following TAVI changes dramatically among different registries, ranging from nearly 4 % to more than 57%.,
Part of this wide heterogeneity is due to the adoption of different definition of CI-AKI among different studies, mainly referring to VARC, VARC 2, RIFLE or AKIN criteria., , ,
Interestingly, the incidence of TAVI-induced AKI has decreased over the years, likely reflecting the overall improvement in procedural techniques, increased team expertise and also and the overall less severe baseline patient conditions.
In a recent analysis by Venturi et Al., a reduction in the CI-AKI incidence throughout the different years was observed from 13.3% between 2012 and 2015, and 3.9% both in the third (2015–2017) and fourth biennium (2017–2019) of a prospective analysis (p=0.012).
When taking into account the risk of AKI, it is important to consider that, if from one hand renal damage may occur during TAVI procedure (Table 1), on the other hand, the improved hemodynamic conditions and increased systemic perfusion induced by TAVI may produce beneficial effects on kidney function that may be able to counterbalance the detrimental effects of contrast dye and other possible procedural renal insults.
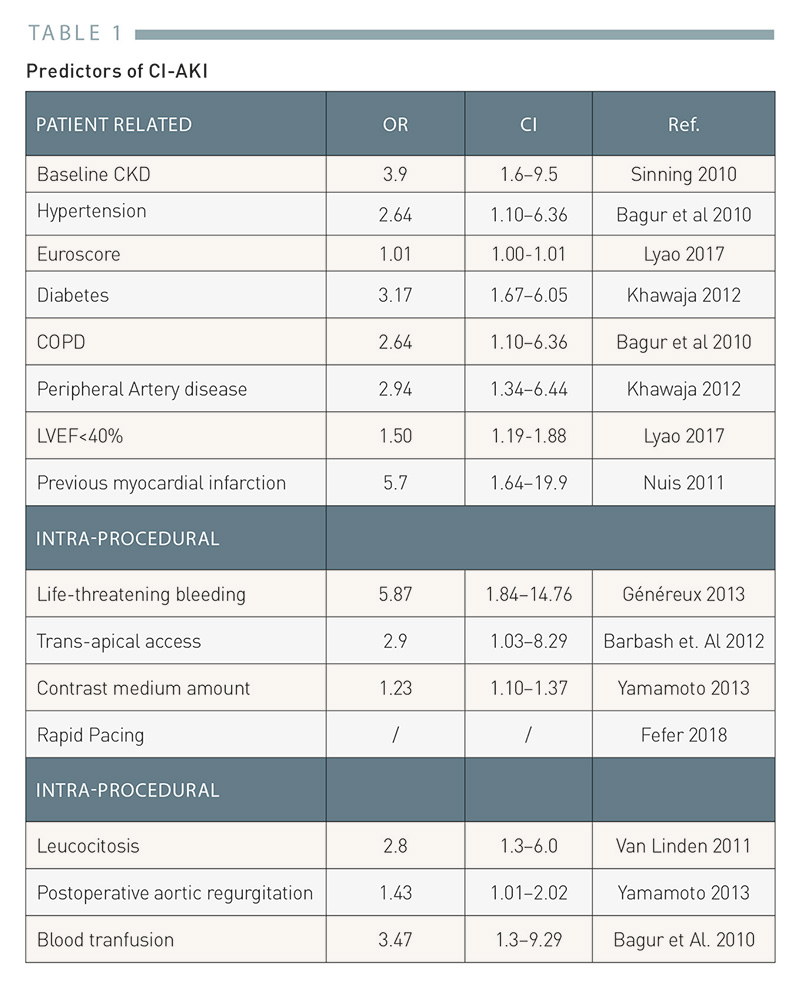
Table 1
Predictors of CI-AKI in TAVI
Notably, Venturi et al. observed that, in a population matched for age, sex and baseline renal function, the procedure type (PCI vs TAVI) was independently associated with CI-AKI. In particular, patients undergoing TAVI had a significantly lower risk of developing CI-AKI than patients undergoing coronary procedures (OR 0.334; 95% CI, 0.193–0.579; p<0.001).
Acute kidney recovery (AKR) defined as eGFR increase > 25% after TAVI up to hospital discharge, was found in more than 25% of patients with pre-existing CKD. Interestingly, Azarbal et al observed that AKR was actually more frequent than AKI (17.8% vs 5.1% of patients) and occurred more frequently in patients with pre-existing CKD. Conversely, patients with diabetes mellitus, anemia, and high STS score tended not to show evidence of AKR .
CI-AKI risk factors
CI-AKI predictors in patients undergoing TAVI can be divided in patient-related, intra-procedural and post-procedural, and their awareness can help clinicians in individuating patients at higher risk. Table 1. Predictors of CI-AKI.
Patient-related CI-AKI risk factors include baseline CKD , hypertension, high EuroSCORE risk score, chronic obstructive pulmonary disease and DM . Peripheral arterial disease is associated with a higher CI-AKI risk, likely due to increased chance of cholesterol embolization but also to the presence of atherosclerotic renal disease which may contribute to contrast medium damage. In addition, left ventricular ejection fraction and previous myocardial infarction have been associated with CI-AKI , .
Among the procedural predictors of CI-AKI, life-threatening bleeding has been reported as strong risk factor. Possible causes are the hemodynamic instability with hypotension and renal hypoperfusion following major bleeding and the pro-inflammatory status associated with blood transfusion. Therefore, in high-risk TAVI candidates it is paramount to minimize the risk of vascular access-related bleeding and hypovolemic status. Recently, the routine use of ultrasound-guidance for TAVI transfemoral vascular access was associated with significantly lower risk of access-related complications and bleeding and may thus be considered the strategy of choice in high risk patients.
Access route is also associated with CI-AKI, with transapical access (TA) being significantly associated with AKI compared with transfemoral TAVI (OR 2.9).
The association between contrast medium amount dose and CI-AKI, is still under debate in TAVI patients, with some studies showing a dose-dependent relation , while others not confirming this data , . Table 1reports the most frequently reported factors associated with CI-AKI in patients undergoing TAVI.
CI-AKI impact on TAVI outcomes
The occurrence of CI-AKI is associated with worse outcomes and, in particular, with higher short term mortality (HR: 30 days: 2.12, 95% CI: 1.59-2.83). In particular, the severity of CI-AKI is associated with 30-day adverse outcome, especially when RRT is needed (OR for 30 days mortality: stage 1: 3.41, 95% CI: 1.89-6.13, stage 2: 4.0, 95% CI: 0.43-36.97, stage 3: 11.02, 95% CI: 5.68-21.38)., .
CI-AKI impacts also on long-term survival (7-year mortality: HR 1.71; 95% CI 1.30 to 2.25) . However, some evidences show no differences when comparing outcome of patients survived at the first year after TAVI, (HR, 0.7; 95%CI, 0.2-2.1; P = 0.57), suggesting that the main impact of this phenomenon is higher in the first months.
CI-AKI after TAVI is also associated with increased readmission (30 days re-admission: HR, 1.23; 95% CI, 1.05-1.44) length of stay (11.7 days ± 8.7 vs 8.7 days ± 5, = 0.004), cerebrovascular accidents (OR 1.92, 95% CI 1.23-2.98, p= 0.004) and need for RRT (OR 22.36, 95% CI 11.88-42.12, P = 0.0002).
The proportion of TAVI patients requiring RRT after CI-AKI varies widely among different studies, and trended to decrease over the years, likely due to improvement in operative and post-operative care. However, except for RRT, little data exist about long term implications of post-TAVI CI-AKI on renal function.
Interestingly, Nijenhuis et al. observed a recovery in kidney function among patients experiencing AKI at 3 months of follow up and a further improvement of eGFR in patients who had AKR.
CI-AKI prevention in patients undergoing TAVI
For high risk cases, different approaches aiming at minimizing contrast volume have been proposed.
In particular, an ultra-low and zero-contrast experiences have been recently reported in the pre-TAVI assessment (Figure 4, Figure 5) .
.png)
Figure 4
Technique for ultra-low/zero-contrast TAVI procedure.
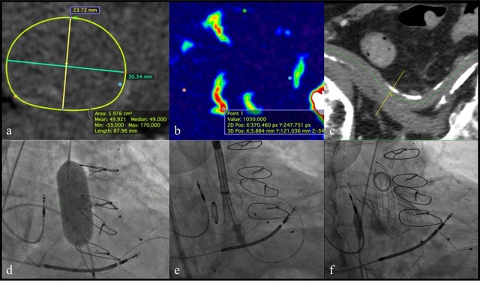
Figure 5
A patient with advanced CKD (eGFR 24 ml/min/1.73m2) and severe aortic stenosis treated with zero-contrast TAVI procedure at our Institution in Verona. Pre-TAVI valve sizing (a, b) and vascular access (c) assessment was based on contrastless CT body weight and surface. Valvuloplasty was performed before TAVI (d). Multiple pigtail catheters at the bottom of the coronary sinuses (arrows) were helpful in guiding the positioning of the valve without use of iodinated contrast (e-f).
Arrigo et al reported an ultra-low contrast TAVI technique in which valve sizing was based on echocardiography (TT/TE), aortography, presence calcification on fluoroscopy, as well as weight and height of the patient. In all cases a self-expandable valve was chosen to minimize annular rupture risk and in borderline measures an oversizing was favored. Access route was decided accordingly to ileo-angiography when available. A single contrast injection of 1 to 3 ml confirmed the correct position of the pigtail catheter, used as the marker for valve deployment at the annulus. After deployment a post dilation was performed in case of relevant aortic regurgitation according to echocardiography assessment.
Recently Castriota et al. reported a zero-contrast TAVI series in which the procedural planning was based on transesophageal echocardiography and on the assessment of annular calcification at the fluoroscopy. In addition, a no-contrast CT and CO2 peripheral angiography were performed: the CT localized and quantified the degree of aortic calcification and the amount and distribution of calcium in iliofemoral vessels; the CO2 angiography assessed peripheral vessels diameters and patency. The access strategy was planned combining both these techniques. No major vascular complications were observed. During TAVI procedure, 3 different pig-tail catheters were positioned in the aortic root for each coronary cusp to better orientating the valve positioning and deployment.
Intravascular volume expansion remains paramount in the setting of TAVI, adopting LVEF or EDP tailored hydration protocols.
The efficacy of forced diuresis with matched hydration with the RenalGuard system in the setting of TAVI is under debate. First open label or observational studies showed a positive effect of this technique (2.7% of Ci-AKI in the therapy group vs 26.7% in the control group, OR 0.06, p<0.001) , suggesting a central role of contrast medium amount in the development of CI-AKI., However, a recent TRIAL (REDUCE-AKI) involving 136 patients randomized to standard hydration (sham group) or to RenalGuard matched hydration with forced diuresis (active group) found no statistically significant differences between the two group (25% in the active group vs. 19.1% in the sham group, p = 0.408). Moreover, a significant increase in long-term mortality was noted in the treatment group (27.9% vs. 13. 2% HR 3.744, 95% CI 1.51-9.28; p= 0.004). These findings reinforce the hypothesis of a multifactorial genesis of CI-AKI in TAVI patients, with a small role of contrast medium.
Pre-TAVI computed tomography angiography and risk of CI-AKI
Computed tomography angiography (CT) is an essential step of TAVI work-up. Nonetheless, contrast media administered during CT may worsen renal function especially in patients with pre-existing CKD with untreated severe aortic stenosis.
Most TAVI centers perform CT assessment in a staged fashion to avoid a contrast medium overload. Nevertheless, new-generation contrast-enhanced CT dual-source CT and dedicated protocols offer sufficient imaging data with low dose of contrast media.,
Moreover, a recent randomized control trial involving 74 CKD (eGFR < 60) patients compared a short, 1-h hydration with sodium bicarbonate to conventional 24-h, high-volume sodium chloride hydration for the prevention of CI-AKI following pre-TAVI CT, finding the non-inferiority of the short hydration arm.
TAVI and bystander coronary artery disease
Severe aortic stenosis is often associated with CAD requiring proper assessment and often interventions. Management of CAD in patients undergoing TAVI may have important implication on renal function being associated with longer procedural time, larger amount of contrast medium and increased risk of procedural complications.
In particular, the best management and timing of coronary angiography/PCI in patients undergoing TAVI is still matter of intense debate. The rationale of performing coronary angiography/PCI in a staged fashion before TAVI is about getting an easier access to the coronary ostia, reducing the risk of ischemic complications during valve implantation and reducing the amount of contrast dye staging the coronary and TAVI procedure. However, the implications on renal function of performing coronary procedures in staged vs concomitant fashion during TAVI have not been clarified and require further investigations.
Importantly, TAVI work up in patients with CKD and especially contrast-based procedures (e.g. CT angiography, coronary angiography, PCI) should be performed in high-volume TAVI centers with dedicated protocols and experience in performing ultra-low/zero contrast procedures. Coronary angiography and/or PCI could be postponed after TAVI in selected patients in order to minimize the risk of AKI.
Transcatheter mitral valve interventions in patients with CKD
Percutaneous mitral valve interventions emerged as a valuable option in patients with unfavorable risk benefit ratio for mitral surgery. The interplay between renal function and mitral valve function has been less intensively investigated compared to the aortic valve stenosis.
Percutaneous edge-to-edge mitral valve repair is a contrast-free procedure. Therefore it is rarely associated with AKI. Length of procedure (probably related to prolonged hypotension) and peripheral vascular disease have been associated with post-MitraClip AKI.
Mitral valve repair, especially in patients with advanced heart failure, reversing the hemodynamic features associated with adverse cardiac remodeling, may improve the renal function. As observed for TAVI, current evidence suggests that the correction of severe mitral valve regurgitation may be associated with improvement in overall cardiac and renal function. Notably, the observed improvement in eGFR was more significant in patients with baseline more severe CKD. A combined analysis of the EVEREST and REALISM studies demonstrated that after MitraClip repair there was a modest improvement in kidney function before hospital discharge and, at 1 year, a significant improvement in eGFR was observed in patients with baseline stage 3-5 CKD but not in those with baseline stage 1-2 CKD.
The impact of CKD on outcome post transcatheter mitral valve repair
The presence of CKD is associated with worse outcome in patients undergoing transcatheter mitral valve interventions. In particularly, CKD increases in hospital mortality (OR 1.29, 95%CI 1.01-1.65, p=0.04) and the risk of rehospitalization (OR 1.16, 95%CI 1.04-1.29, P=0.006) of patients who underwent edge-to-edge mitral valve repair, .
A subanalysis of the EVEREST trial performed on 886 patients revealed that the degree of pre-existing CKD was associated with long-term mortality, with patients with severe CKD (eGFR <30 ml/min/1.73 m) having the worse prognosis (HR 3.80, 95%CI 2.38-6.05) and patients with eGFR between 30 and 60 ml/min/1.73 mhaving better outcome but still worse survival compared with patients with preserved renal function (HR 2.35, 95%CI 1.73-3.19). Similarly, an analysis from the GRASP registry reported significantly lower event-free survival rate for patients with CKD (eGFR 2.39, 95%CI 1.19-4.78, p=0.014). Therefore, an appropriate patient selection is essential based on survival expectancy, especially in patients with severe CKD (eGFR <30 ml/min/1.73m) and multiple comorbidities
Personal perspective - Flavio Ribichini
The preservation of renal function is key in the long-term outcome of patients undergoing myocardial revascularisation procedures, and myocardial revascularisation procedures strongly determine survival of patients with CKD. Patients with CKD require strict cardiovascular control and a conscious evaluation of revascularisation strategies, independently of the clinical presentation of the CAD. Furthermore, preventive measures to avoid worsening of GFR and optimisation of medical treatment are essential to balance risks and benefits of any revascularisation strategy in this delicate sub-set of patients.
All patients scheduled for PCI should be correctly informed about the potential benefits and risks of the procedure, including the risk of a procedure-related worsening of the renal function. Patients with a known CKD are exposed to a particularly higher risk of suffering further impairment of the renal function, and eventually the need for permanent dialysis, despite the conscious implementation of preventive measures.
The risk-benefit balance of myocardial revascularisation in this context has extreme importance. Therefore, both the indication of the most appropriate means of revascularisation as well as the application of effective preventive measures that could avoid or reduce the risk of a collateral renal damage secondary to the revascularisation procedures are essential.
Percutaneous interventions in patients with advanced CKD and diffuse or calcified CAD require adequate lesion preparation, the use of rotational atherectomy before stenting may be necessary, and intravascular ultrasound afterwards to optimise results should be considered.
Randomized trials comparing PCI vs CABG in complex CAD scenarios, such as three-vessel disease or left main, showed no significant difference in the risk of mortality or myocardial infarction at 5 or 10 years of follow up. However, data on patients with CKD are still insufficient to define the best revascularization technique in this setting and further investigations are warranted.
Last, but not least, the long-term cardiorenal protection is crucial. This involves statins, strict blood pressure control to a target of less than 130/80mmHg and blockade of the renin-angiotensin system.
Structural valve interventions are an essential component in contemporary interventional practice. Patients with CKD undergoing TAVI are at increased risk of adverse outcome compare with patients without CKD. Nonetheless, TAVI has the potential to improve the kidney function yielding a consistent survival benefit in this subgroup.
Patients undergoing TAVI with bystander CAD and CKD require often complex procedures and are at high risk of periprocedural worsening of the renal function.
Patients with CKD and CAD represent a unique clinical setting that requires further intense investigation to establish standards of care in areas of practice so far unknown because of the lack of comparative data. The real need for revascularization in TAVI patients, and whether this should be performed before or after the valve replacement remains to be defined. Furthermore, the added value of dedicated devices (rotational atherectomy, lithotripsy, intravascular imaging), or specific drugs, or new surgical techniques, are all important issues under investigation.