Left atrial appendage occlusion
Summary
Atrial fibrillation is the most common arrhythmia worldwide, with a lifetime incidence approaching 25% and an ever-increasing prevalence in an aging society. It is projected that by the year 2050, 6 to 12% of the world’s population will be diagnosed with atrial fibrillation, and 18 million will be affected in Europe by the year 2060. The most devastating consequences of atrial fibrillation are embolic cerebrovascular accidents. While anticoagulation strategies result in significant reductions in embolic events, they are not without limitations and complications, namely bleeding. In patients with atrial fibrillation, the vast majority of thrombus originates in the left atrial appendage. Exclusion of the left atrial appendage has been demonstrated to be as effective as oral anticoagulation with Vitamin K antagonists in the prevention of disabling strokes and obviates the need for chronic anticoagulant therapy.
The field of transcatheter endovascular left atrial appendage occlusion (LAAO) has expanded exponentially since its inception two decades ago and is potentially emerging as the anti-embolic treatment of choice for patients with nonvalvular atrial fibrillation pending the results of highly anticipated trials. While the Watchman and Amplatzer Amulet devices dominate the transcatheter percutaneous LAAO market shares in both Europe and America, the structural proceduralist will soon be able to choose from multiple device options to accommodate the wide variation of LAA morphologies present in the human population. LAAO devices are currently indicated in patients with nonvalvular atrial fibrillation with contraindications to anticoagulation. While no procedure is risk-free, device iteration has resulted in improved safety and versatility. This chapter reviews the underlying concepts involving atrial fibrillation, outlines anticoagulation strategies for thromboembolism prevention, and describes the current state and future directions of left atrial appendage occlusion.
Introduction
Atrial fibrillation is an extremely common condition with a prevalence of approximately 0.4% and a strong association with age and male sex. The age-adjusted incidence approaches 5% per year in patients greater than 80 years of age, and prevalence in this population is approximately 9%. The lifetime risk of developing atrial fibrillation is approximately 1 in 4.
Mechanistically, atrial fibrillation is characterized by uncoordinated and uncontrolled activation of atrial tissue. Although numerous classification schemes exist, the most commonly employed classification system refers to timing and duration of the arrhythmia (Figure 1). Paroxysmal atrial fibrillation refers to episodes lasting usually less than 48 hours, although individual paroxysms may last up to 7 days. Persistent atrial fibrillation either lasts longer than 7 days or requires electrical/chemical cardioversion to restore sinus rhythm. Atrial fibrillation lasting greater than one year is either considered long-standing persistent if a rhythm control strategy is pursued or permanent if no further rhythm control methods are planned. ,
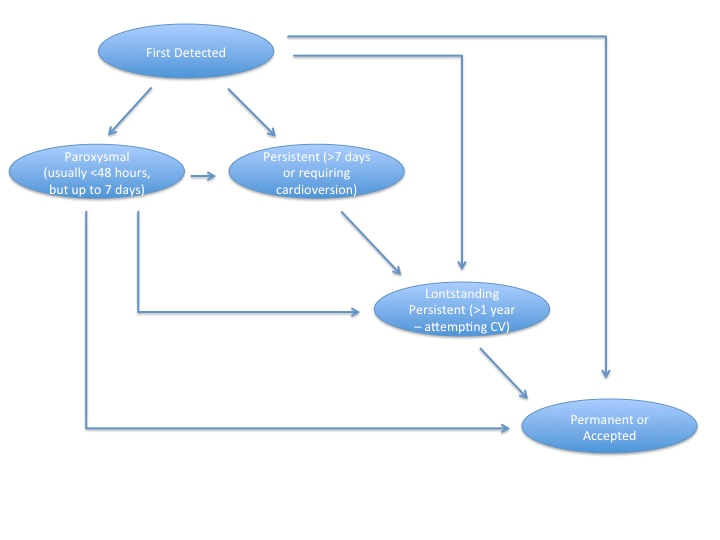
Figure 1
Classification of atrial fibrillation
Treatment and Prevention
Medical Therapies
Treatment strategies for atrial fibrillation center around two concepts: management of symptoms and prevention of complications. In terms of symptoms, management can either focus on rate or rhythm control. Rate control strategies include beta-blockers, calcium channel blockers, digoxin, and in refractory cases, atrioventricular node ablation with pacemaker implantation. Rhythm control strategies include medical therapies such as sotalol, dofetilide, amiodarone, dronedarone, electrical cardioversion or pulmonary vein isolation.
Regardless of the treatment strategy for rate or rhythm control, treatment efforts must also focus on prevention of thromboembolic events. Atrial fibrillation increases the risk of cerebral ischemic events 5-fold across the entire spectrum of age, and it accounts for approximately 1.5% of events in patients under age 50 to greater than 20% of events in patients greater than age 80. Regardless of whether it is paroxysmal or permanent, the annual rate of cerebral ischemic events in patients with atrial fibrillation approaches 4.5% per year. In fact, even subclinical atrial fibrillation lasting 6 minutes or longer is associated with an increased risk of ischemic events.
Although the overall risk of cerebral ischemic events in patients with atrial fibrillation is understood on the population level, it is both practical and important to make attempts at individualizing the risk for a particular patient. A number of risk models have been proposed. The first is the CHADS2 score. In this model, a patient is assessed according to 5 established risk factors (Table 1) including age >75, history of hypertension, history of congestive heart failure, diabetes, and prior embolic event. Each risk factor is worth one point with the exception of a prior embolic event, which is worth two points. The resultant score ranges from 0-6 and corresponds to a significant increase in risk for each incremental score. The absolute risk varies by series. In one study of 1733 patients with atrial fibrillation not on warfarin, the annualized risk of cerebral ischemic events ranged from 1.9% for a CHADS2 score of 0 to 18.2% for a CHADS2 score of 6. In another, larger series of 11,526 patients with atrial fibrillation not on warfarin, the annualized risk of events ranged from 0.49% for a CHADS2 score of 0 to 6.9% for a CHADS2 score of 6. Although these two studies highlight variability in cerebral ischemic event risk across different populations, the unifying concept that higher CHADS2 scores portend higher event risk remains true.
Table 1. CHADS2 Score and Associated cerebral event Risk,
| CHADS2 Score | Annual Risk of Cerebral Events (± 95% CI) | Treatment Recommendation |
|---|---|---|
| 0 | 0.49% (0.30-0.78) 1.9% (1.2-3.0) |
Aspirin 325mg daily |
| 1 | 1.52% (1.19-1.94) 2.8% (2.0-3.8) |
Aspirin 325mg daily or Warfarin – target INR 2-3 |
| 2 | 2.50% (1.98-3.15) 4.0% (3.1-5.1) |
Warfarin – target INR 2-3 |
| 3 | 5.27% (4.15-6.70) 5.9% (4.6-7.3) |
Warfarin – target INR 2-3 |
| 4 | 6.02% (3.90-9.29) 8.5% (6.3-11.1) |
Warfarin – target INR 2-3 |
| 5 | 6.88% (3.42-13.84)* 12.5% (8.2-17.5) |
Warfarin – target INR 2-3 |
| 6 | 6.88% (3.42-13.84)* 18.2% (10.0-27.4) |
Warfarin – target INR 2-3 |
| Risk factors include: Age > 75 years old, Diabetes, Hypertension, Congestive Heart Failure (1 point each) and Prior thromboembolic event (2 points) for a total score of 0-6. *In this study, patients with CHADS2 scores of 5 and 6 were grouped together. |
||
Although the CHADS2 score is well studied and commonly applied, it is uncertain whether this score adequately stratifies low risk patients. This point is highlighted by the fact that even low risk patients (those with CHADS2 scores of 1) can benefit from oral anticoagulation with warfarin or the non-vitamin K oral anticoagulant medications discussed below. Furthermore, greater emphasis on certain risk factors and the addition of other known risk factors for cerebral ischemic events may further refine risk stratification. The CHA2DS2VASc score (Table 2) is a 9-point scale incorporating the factors in the CHADS2 system (congestive heart failure, hypertension, age >75, diabetes and prior stroke x2) placing increased emphasis on age (65-74 merits 1 point while ≥75 merits 2 points). Additionally, it adds vascular disease and female gender as risk factors. Based on an analysis of 1084 patients Lip et al., validated this risk model and demonstrated incremental risk of embolic events with a rising score (Table 2). This score has been further validated in a trial of over 7000 patients. While the CHA2DS2VASc score is a more comprehensive system, and it has become the standard in assessing thromboembolism risk, it is worth recognizing that small numbers at either end of the risk spectrum may under- or overestimate absolute risk.
Table 2. CHA2DS2VASc Score and associated cerebral event risk (n=1084).,
| CHA2DS2VASc Score | Annual Risk of Cerebral Event (±95% CI) | Treatment Recommendation |
|---|---|---|
| 0 | 0% (0-0) | Aspirin alone or no therapy |
| 1 | 0.6% (0-3.4) | Aspirin (75-325mg) or oral anticoagulation |
| 2 | 1.6% (0.3-4.7) | Oral anticoagulation |
| 3 | 3.9% (1.7-7.6) | Oral anticoagulation |
| 4 | 1.9% (0.5-4.9) | Oral anticoagulation |
| 5 | 3.2% (0.7-9.0) | Oral anticoagulation |
| 6 | 3.6% (0.4-12.3) | Oral anticoagulation |
| 7 | 8.0% (1.0-26.0) | Oral anticoagulation |
| 8 | 11.3% (0.3-48.3) | Oral anticoagulation |
| 9 | 100% (2.5-100) | Oral anticoagulation |
| Risk factors include: Prior embolic event (2 points), Age > 75 (2 points), Age 65-74 (1 point), congestive heart failure (1 point), hypertension (1 point), diabetes (1 point), vascular disease (1 point), female gender (1 point). p=0.003 for trend. | ||
It is also important to balance the risk of cerebral ischemic events against the risk of bleeding. Numerous algorithms have been proposed, but the most commonly utilized is the HAS-BLED score (Table 3), incorporating hypertension, abnormal liver/kidney function, prior stroke, prior bleeding, labile INRs, elderly and drug/alcohol abuse on a 9 point scale. Scores of ≥ 3 are associated with increased bleeding risk, but it is important to note that often, similar factors (such as age and hypertension) increase both the bleeding risk and the risk of cerebral ischemic events, and often, the risk related to bleeding is outweighed by the risk of stroke. Decision algorithms designed to balance these risks have been proposed , and both the 2012 updated European guidelines and the 2014 ACC/AHA/HRS guidelines recommend thoughtful anticoagulation with close monitoring in patients at elevated risk for bleeding. ,
Table 3. HAS-BLED Score predicting bleeding risk.
| Table 3a – HAS-BLED | Table 3b – Annual Bleeding Risk | ||
|---|---|---|---|
| Description | Points | Cumulative Points | Bleeding Risk |
| (H)ypertension | 1 | 1 | 1.02% |
| (A)bnormal liver function or renal function | 1 | 2 | 1.88% |
| 3 | 3.74% | ||
| (S)troke | 1 | 4 | 8.70% |
| (B)leeding | 1 | 5 | 12.50% |
| (L)abile INR | 1 | 6 | Insufficient Data |
| (E)ldery (Age > 65) | 1 | 7 | Insufficient Data |
| (D)rug use or alcohol use | 1 | 8 | Insufficient Data |
| 9 | Insufficient Data | ||
Multiple anticoagulation strategies to prevent cerebral ischemic events have been studied (Table 4), including aspirin, clopidogrel, warfarin and the direct oral anticoagulants: dabigatran, rivaroxaban, apixaban and edoxaban. Both aspirin and warfarin have been compared to placebo. In 1999, Hart et al. published a meta-analysis of studies comparing aspirin to placebo, warfarin to placebo and aspirin to warfarin in patients with atrial fibrillation. Across the studies comparing aspirin to placebo with the primary endpoint of cerebrovascular accident, therapy with aspirin resulted in a relative risk reduction of 22% and an absolute risk reduction of 1.5% per year respectively for primary prevention and 2.5% per year for secondary prevention. In the studies comparing warfarin to placebo, warfarin resulted in a relative risk reduction of 62% with absolute reductions of 2.7% per year for primary prevention and 8.4% per year for secondary prevention. In 5 studies comparing aspirin to warfarin, warfarin resulted in a 34% relative risk reduction, proving superior to aspirin in the reduction of cerebral events; however, it should be noted that the risk of both intracranial and extracranial hemorrhage was higher with warfarin compared to aspirin.
Table 4. Anticoagulation options for patients with atrial fibrillation.
| Anticoagulation Strategy | Comparison Group | Study Name | Cerebral Events Risk | Comments |
|---|---|---|---|---|
| Aspirin | Placebo | Hart et al. (Meta-analysis of 6 trials) | Relative Risk Reduction 22% (CI 2-38) | Superiority of aspirin over placebo |
| Warfarin | Placebo | Hart et al. (Meta-analysis of 6 trials) | Relative Risk Reduction 62% (CI 48-72) | Superiority of warfarin over placebo |
| Aspirin | Hart et al. (Meta-analysis of 5 trials) | Relative Risk Reduction 36% (CI 14-52%) | Superiority of warfarin over aspirin | |
| Dual Antiplatelet (Aspirin and clopidogrel) | Warfarin | Connolly et al. ACTIVE W | Relative Risk 1.44 (95% CI 1.18-1.76) | Trial stopped early due to benefit with warfarin |
| Aspirin | Connolly et al. ACTIVE A | Relative Risk 0.72 (95% CI 0.62-0.83) | Bleeding risk 1.57 (95% CI 1.29-1.92) | |
| Dabigatran 110mg twice daily | Warfarin | Connolly et al. RE-LY | Relative Risk 0.91 (95% CI 0.74-1.11) | Bleeding risk lower with dabigatran |
| Dabigatran 150mg twice daily | Warfarin | Connolly et al. RE-LY | Relative Risk 0.66 (95% CI 0.53-0.82) | Bleeding risk similar between groups |
| Rivaroxaban 20 mg daily | Warfarin | Patel et al. ROCKET-AF | Relative Risk 0.79 (95% CI 0.66-0.96) | Similar overall bleeding, less intracranial/fatal |
| Apixaban 5 mg twice daily | Warfarin | Granger et al. ARISTOTLE | Relative Risk 0.79 (95% CI 0.66-0.95) | Less overall bleeding and all-cause mortality |
| Edoxaban 30 mg daily | Warfarin | Guigliano et al. ENGAGE-AF | Relative risk 1.07 (95% CI 0.87-1.31) | Less bleeding and non CV death |
| Edoxaban 60 mg daily | Warfarin | Guigliano et al. ENGAGE-AF | Relative risk 0.79 (95% CI 0.63-0.99) | Less bleeding and non CV death |
Clopidogrel has also been studied in patients with atrial fibrillation. The Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE) trials evaluated the efficacy and safety of dual antiplatelet therapy in patients with atrial fibrillation. The ACTIVE W trial evaluated dual antiplatelet therapy versus warfarin in 6726 patients with atrial fibrillation and at least one other risk factor for thromboembolic events with a primary endpoint of cerebral events, non-cerebral embolic events, myocardial infarction or vascular death. This study was stopped early, as therapy with dual antiplatelet therapy was associated with a significantly higher event rate (relative risk 1.44, 95% CI 1.18-1.76, p=0.003) compared to warfarin alone.
In the ACTIVE A trial, 7554 patients with atrial fibrillation at increased risk of stroke who were considered unsuitable candidates for warfarin therapy were randomized to aspirin plus clopidogrel versus aspirin alone. Over a mean of 3.6 years of follow-up, patients treated with dual antiplatelet therapy had a significantly lower risk of the composite primary endpoint consisting of cerebral event, non-cerebral embolic event, myocardial infarction or vascular death (relative risk 0.89, 95% CI 0.81-0.98, p=0.01) at the cost of an increased risk of bleeding (relative risk 1.57, 95% CI 1.29-1.92, p<0.01). As a result of these and other studies, dual antiplatelet therapy has not gained widespread acceptance for thromboembolic prevention in patients with atrial fibrillation except for patients in whom warfarin is contraindicated.
Although long-term anticoagulation with warfarin is efficacious, numerous drawbacks exist. The narrow therapeutic window of warfarin is a delicate balance between lack of efficacy and a significantly elevated risk of bleeding, therefore requiring frequent blood tests. Additionally, numerous food and drug interactions exist, thus making chronic management of multisystem disease difficult. Finally, in patients at risk for falling, anticoagulation itself may incur more risk than the thromboembolic event it is prescribed to prevent.
Highlighting these issues, warfarin therapy has been associated with bleeding rates upwards of 10% per year, leading to a regulatory “black box warning”. Additionally, up to 40% of patients with atrial fibrillation have contraindications to anticoagulation therapy. Warfarin is often underutilized or associated with difficulties in finding appropriate dosage.
Among patients who are at moderate or high risk for ischemic stroke and considered good candidates for warfarin therapy, it is prescribed in only 40% of patients, and even in the controlled Stroke Prevention Using an Oral Thrombin Inhibitor in Atrial Fibrillation (SPORTIF III and V) trial settings, up to 30% of patients were subtherapeutic and 15% supratherapeutic on warfarin. Finally, in a study of 41900 patients with chronic atrial fibrillation, only 50% of patients treated with aspirin and 70% of patients treated with warfarin remained on this therapy at one year, further highlighting difficulties with anticoagulation.
Despite these issues, vitamin K antagonists remain the most commonly employed medical thromboembolic prevention in atrial fibrillation, particularly in patients at elevated risk of thromboembolism. According to the ESC guidelines, assessing the individualized risk of cerebral events in patients with atrial fibrillation and the risks of chronic therapy with warfarin is paramount. , As outlined in Table 2, patients at low risk for cerebral events (CHA2DS2VASc score of 0) can be treated with aspirin alone or no therapy at all. Those at higher risk should be treated with oral anticoagulation unless otherwise contraindicated due to excessive bleeding risk. , ,
The non-vitamin K oral anticoagulants, dabigatran, rivaroxaban and apixaban have gained considerable interest for thromboembolic event prevention. The first such agent was dabigatran, studied in the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) Trial evaluating 18,113 patients with atrial fibrillation and an increased risk of stroke (mean CHADS2 score of 2). Patients were treated with warfarin or dabigatran (110 or 150 mg twice daily) with a primary endpoint of cerebral or systemic embolic events in a non-inferiority design. Both doses of dabigatran proved non-inferior to warfarin; however, dabigatran at 150 mg twice daily proved superior to warfarin with regard to both the primary endpoint (relative risk 0.66, 95% CI 0.53-0.82, p<0.001) as well as net clinical benefit, including the primary endpoint, bleeding and death (relative risk 0.91, 95% CI 0.82-1.00, p=0.04). This benefit appears to be even greater in patients with poor anticoagulation control with warfarin, but it should be noted that medication discontinuation rates were significantly higher with either dose of dabigatran than with warfarin at both one year (14.5% dabigatran 110 mg, 15.5%, dabigatran 150 mg, 10.2% warfarin, p<0.01) and two-years (20.7% dabigatran 110 mg, 21.2%, dabigatran 150 mg, 16.6% warfarin, 0<0.01).
Rivaroxaban was studied in the 14,226 patient Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for the Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Patients were randomized to either warfarin targeted to an INR of 2.0-3.0 or rivaroxaban 20 mg daily (15 mg daily for creatinine clearance 30-49 ml/min) with a primary composite endpoint of stroke and systemic embolism. In the primary, as-treated analysis, there were similar rates of stroke and systemic embolism (1.7%/year rivaroxaban vs. 2.2%/year warfarin, HR 0.79, 95% CI 0.66-0.96, p<0.001 for non-inferiority). There were similar rates of bleeding between the two groups, but intracranial (0.5% vs. 0.7%, p=0.02) and fatal bleeding (0.2% vs. 0.5%, p=0.0.03) were both significantly lower in the rivaroxaban treated patients.
The Apixaban for Reduction of Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial randomized 18,201 patients with atrial fibrillation and at least one risk factor for cerebral embolic events to warfarin with a goal INR of 2.0-3.0 or apixaban 5 mg twice daily with a primary endpoint of stroke or ischemic embolism over a median 1.8 year follow-up. In this study, the primary endpoint occurred in 1.27%/yr. in the apixaban group compared to 1.6%/year in the warfarin group (HR 0.79, 95% CI 0.66-0.95, p<0.001 for non-inferiority, p=0.01 for superiority). Apixaban was also associated with reductions in major bleeding (2.1%/year apixaban vs. 3.1 %/year warfarin, p<0.001) and all-cause mortality (3.5% apixaban vs. 3.9% warfarin, p=0.047).
The Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) randomized 21,105 patients with nonvalvular atrial fibrillation to warfarin (n=7036) or edoxaban at either 60 mg (n=7035) or 30 mg (n=7034) with a primary efficacy endpoint of stroke or systemic embolic event and a primary safety endpoint of major bleeding. In this noninferiority designed trial, stroke or systemic embolism occurred in 1.50% patients/year with warfarin versus 1.18% patients/year with edoxaban 60 mg (HR 0.79; 97.5% CI 0.63-0.99; p<0.001 for noninferiority) and 1.61% patients/year with edoxaban 30 mg (HR1.07; 97.5% CI, 0.87-1.31; p=0.005 for noninferiority).
Bleeding events occurred at a rate of 3.43% patients/year with warfarin versus 2.75% patients/year with edoxaban 60 mg (HR 0.80; 95% CI, 0.71 to 0.91; p<0.001) and 1.61% patients/year with Edoxaban 30 mg (HR 0.47; 95% CI, 0.41 to 0.55; P<0.01). Additionally, non-cardiovascular death, a prespecified secondary endpoint, occurred in 3.17% patients/year with warfarin versus 2.74% with edoxaban 60 mg (HR 0.86; 95% CI, 0.77 to 0.97; p=0.01), and 2.71% with edoxaban 30 mg (HR 0.85; 95% CI, 0.76 to 0.96; p=0.008).
While target specific oral anticoagulants appear to be both safe and effective for patients with non-valvular atrial fibrillation, it is important to note that the clinical trials involve carefully selected patients, and these agents will require post-market analysis to establish their relative utility in more general populations. Additionally, the pivotal trials were conducted against warfarin, so head-to-head comparisons are accordingly lacking. Nevertheless, the preponderance of evidence suggests that oral anticoagulation is superior to antiplatelet therapy alone, and these newer agents have advantages over warfarin in appropriately selected patients.
The Origins and Evolution of LAAO
In 1946, William Dock applied the thought of removing thrombus together with its site of origin to prevent recurrent systemic emboli to the specific patient population with rheumatic heart disease, mitral stenosis, atrial fibrillation, and recurrent arterial emboli. He proposed left atrial appendage resection as a solution. In 1949, John L. Madden successfully performed left atrial appendage resection on two patients with rheumatic heart disease, mitral stenosis, and atrial fibrillation with peripheral arterial and aortic emboli, respectively. He observed that in over 90 percent of cases of rheumatic heart disease, the thrombi were found in the left atrium, particularly the appendage. This preliminary observation would be observed decades later in a meta-analysis conducted by Blackshear and Odell. They noted in their review that 201 of 222 (91%) of nonrheumatic atrial fibrillation-related left atrial thrombi originated in the left atrial appendage. In comparison, 254 out of 446 (57%) of patients with rheumatic atrial fibrillation had thrombi originating from the left atrial appendage. This observation had profound lasting clinical implications, as LAAO is performed in patients with nonvalvular atrial fibrillation. With this knowledge, surgical excision of the left atrial appendage occurred with concomitant cardiac surgery such as mitral valve replacement and repair and coronary artery bypass graft surgery, though appendage obliteration was not standard fare for every cardiac surgery case and highly dependent on surgeon preference. The maze procedure developed by Cox for atrial fibrillation entails multiple planned incisions in the right and left atria and complete right and left atrial appendage excision with suture closure. The Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO) device was the first transcatheter endovascular device developed in 2000, which fueled many studies and investigations, ultimately culminating in the approval and widespread application of percutaneous left atrial appendage occlusion.
Left Atrial Appendage Anatomy
The LAA is an embryonic remnant of the primordial left atrium, with a varied range of several architectural facets including ostial diameter, depth, and shape. This structure stretches across the anterior and lateral walls of the left atrium, nestled in the atrioventricular groove inferior to the pulmonary outflow trunk and superior to the left ventricle. The LAA tip is directed anterosuperiorly and rests along the left border of the right ventricular outflow tract. The LAA is a thin-walled structure (1 mm) lined with thick (> 1 mm) trabeculated pectinate muscles, and distinguishes itself embryologically from the rest of the smooth-walled left atrium. The ostia of the LAA have a number of described shapes and diameters, including oval (82%), triangular (7%), semi-circular (4%), and round and water-drop shapes. The body of the LAA has been characterized by CT and MRI and divided into four different morphological categories: chicken wing, cactus, windsock, and cauliflower. The chicken-wing morphology has been shown to be associated with a 79% reduced chance of stroke in patients with atrial fibrillation (after adjusting for CHADS2 score, gender, and type of AF), while the cactus, windsock and cauliflower morphologies is associated with a four-to-eight-fold greater risk of stroke. Patients with persistent AF are more likely to have non-chicken wing LAA morphologies compared with patients with paroxysmal AF. The normal LAA exhibits wide variations in the number of lobes, length, and volume. In an autopsy study of 500 normal hearts, Veinot et al., found that 54% of LAA had two lobes, whereas 23% had 3 lobes and 20% had only one lobe. Volumes ranged from 0.7-19.2 cc, length ranged from 16-51 mm and orifice diameter ranged from 10-40 mm. Patients with persistent AF have been shown to have a higher LAA volume and larger and rounder ostial area, so the duration of AF should be considered whenever embarking on device-sizing selection. The normal LAA exhibits contractility and quadriphasic blood flow; both properties are impaired in patients with atrial fibrillation.
Surgical LAA exclusion
Surgical exclusion of the LAA is the earliest form of LAAO, dating back to the 1940s. Surgical exclusion of the LAAO had been increasingly common when patients with atrial fibrillation undergoing otherwise indicated cardiac surgery despite little published data to support the efficacy of this practice. The first randomized trial to evaluate the safety and efficacy of surgical LAAO was the Left Atrial Appendage Occlusion Study (LAAOS) published in 2005. In this trial, 77 patients with atrial fibrillation and risk factors for cerebral events undergoing coronary artery bypass surgery were randomized 2:1 to LAA exclusion (LAAE) (with sutures or staples) or no treatment. LAAE did not prolong bypass time or increase heart failure, atrial fibrillation, or bleeding incidences. There were 9 LAA tears easily repaired by suture. Of the 52 patients randomized to exclusion, 44 underwent repeat TEE.
There was such a low success rate with LAA closure with sutures (5 out of 11 patients, 45%) that the study converted to using staplers for closure. There was a trend towards more success with staples, with 24/33 (72%) of patients achieving complete closure, though because staples were used later in the study when surgeons were gaining more experience with the procedure, it is hard to dissect which factor contributed more towards success. Interestingly, no statistical significance for occlusion success was detected between the suture and staple groups (p = 0.14). The study was not powered to assess events, but the results suggest suboptimal closure rates despite the employment of multiple techniques. With regards to stroke, 2 patients (2.6%) had periprocedural stroke (one had an ischemic stroke intraoperatively, the other had a transient ischemic attack (TIA) on postoperative day 3), though this study was not powered to determine stroke outcomes. Patients were followed for 13 ± 7 months, and no patient had any stroke in the long-term. The authors conclude that use of a staple device by an experienced surgeon creates a successful (>90%) platform for surgical LAAE. Over a decade ago, meta-analyses investigating the benefit of surgical LAAE yielded conflicting results (this included the LAAOS study) and overall were unable to support the practice, citing incomplete closure (average 55-65%) as the primary driver behind the disappointing results. As a result, surgical LAAE during cardiac surgery for patients with AF was listed as a class IIb recommendation, though this does not deter performance of the procedure in clinical practice.
A more recent effort by Atti et al., to derive a meta-analysis of 12 studies of patients undergoing surgical LAAE versus not during concomitant cardiac surgery (valve and coronary artery bypass grafting surgeries were included) showed that patients with exclusion had lower events of systemic embolism (p < 0.001) and stroke (p < 0.0001). Successful exclusion is heavily influenced by operator experience, technique, and anatomy; this analysis was also unable to decipher which surgical technique yielded best results. In contrast to percutaneous LAAO, questions regarding long-term anticoagulation after surgical exclusion have not yet been answered. The updated 2020 ESC/EACTS guidelines recommend enduring anticoagulation therapy in patients with AF surgery and appendage closure, depending on their CHA2DS2 -VASc score and overall stroke risk (Class I, level of evidence C).
Further bolstering the utility of LAAE during cardiac surgery for patients with AF, more recently in 2021, the Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke (LAAOS III) Study was published. This is a randomized, multicenter trial involving patients with atrial fibrillation (CHA2DS2 -VASc ≥2), in which patients were randomly assigned to undergo left atrial appendage occlusion during cardiac surgery scheduled for another indication. Surgical LAAE methods included the standard and preferred amputation and closure, stapler closure, TEE-confirmed double-layer linear closure, or closure with an approved surgical occlusion device. Patients were expected to adhere to oral anticoagulation post-surgery. The primary outcome assessed was occurrence of stroke or systemic embolism. At 3 years, 77% of the participants continued to receive oral anticoagulation. Stroke or systemic embolism occurred in 114 out of 2379 (4.8%) patients in the occlusion group and in 168 out of 2391 (7.0%) in the no-occlusion group (hazard ratio 0.67, P = 0.001), with an estimated number needed to treat to prevent one stroke over 5 years of 37 (95% CI, 22 to 111). Surgical occlusion had little effect on bypass time and cross-clamp time and did not have a significant effect on the secondary outcomes of death, hospitalization for heart failure, myocardial infarction, or bleeding. LAAOS III suggests that the anti-embolic effects of surgical LAAO are additive to those of oral anticoagulation. The study did not compare surgical LAAO alone against anticoagulation, so surgical LAAO alone cannot be considered a replacement for anticoagulation at this time. The LAAOS III study was not designed to parse out which surgical method of closure may be superior, or whether surgical occlusion was sustained over the long-term. Because surgical exclusion is an extravascular procedure (as compared to endovascular percutaneous occlusion devices), there is speculation this may protect against device-related thrombus and future strokes, although this has never been studied.
Surgical occlusion devices have been studied and are promising alternatives to amputation, sutures, and staples because of the fragility and bleeding of the LAA and the risks that are attendant with incomplete closure. The AtriClip Gillinov-Cosgrove Left Atrial Appendage Exclusion system (Atricure Inc, Westchester, Ohio), or simply known as the AtriClip, was FDA approved in 2010 after results of The Exclusion of Left Atrial Appendage with AtriClip Exclusion Device in Patients Undergoing Concomitant Cardiac Surgery (EXCLUDE). The Atriclip is a clip with 2 stiff, parallel titanium tubes covered with knit-braided polyester sheaths and connected by elastic nitinol springs, delivering uniform pressure over the length of the tubes once the device is deployed epicardially. The AtriClip comes in 4 different custom-fitted sizes (35 mm, 40 mm, 45 mm and 50 mm). EXCLUDE was a nonrandomized, prospective, multicenter designed to assess the safety and efficacy of AtriClip in patients with AF undergoing elective cardiac surgery via median sternotomy. Safety was measured by device-related adverse events at 30 days. Efficacy was determined by verification of flow cessation to the LAA as measured by a combination of intraprocedural TEE and 3-month CTA. There were no damage or tears to the LAA or surrounding anatomy, device migration, or bleeding or repair sutures required in any of the patients. At 1 year, 2 patients had strokes thought to be hypertensive and atherosclerotic in etiology (unrelated to device). Of the 70 patients who underwent successful AtriClip device placement, 67 of those (95.7%) achieved successful exclusion by intraprocedural determination, and of the 61 patients who had follow-up TEE or CT, 60 (98.4%) had complete exclusion.
A corresponding European safety and feasibility study with 34 patients showed successful clip placement with no device-related complications, no stroke, and documented stable clip placement on CT at 3 months, leading to CE mark approval. The AtriClip was further tested in the ATLAS trial, a prospective, randomized, multicenter, unblinded trial comparing patients without documented AF and an elevated CHA2DS2-VASc and HAS-BLED score in a 2:1 manner into those who received LAAE and those who did not during otherwise scheduled cardiac surgery. Efficacy endpoints were: 1) incidence of perioperative events such as stroke, bleeding, myocardial infarction, and death, 2) successful intraoperative LAAE, and 3) composite stroke rates through 365 days in those patients who developed postoperative AF (POAF). The clip was successfully placed in 373 patients, with no peri-procedural sequelae. POAF occurred in 47% of the LAAE group and in 38% of the no LAAE group (p = 0.047). While there was a higher incidence of POAF in the LAAE arm, there was a trend towards lower stroke rate in the LAAE arm, with a 1.1% versus 2.8% perioperative stroke rate and a 3.4% versus 7.0% total thromboembolic rate at 1 year in the LAAE versus no LAAE arm, respectively. Overall, clip placement was procedurally successful 99% of the time, with low adverse events rates (0.3%). POAF development was higher in the LAAE group, without a higher ischemic stroke rate. There was no difference in mortality between the 2 groups at 30 days and 1 year.
Cumulatively, it appears the AtriClip is a safe and effective modality for LAAE. An additional noted benefit of the AtriClip is that it provides acute electrical isolation of the LAA, combining both stroke prevention effects and reduction in incidence of recurrent AF. Set to be completed in 2032, AtriCure has an ongoing superiority trial called the The Left Atrial Appendage Exclusion for Prophylactic Stroke Reduction Trial (LeAAPS), which is an expansion to ATLAS in that it will assess the ability of LAAE (versus no LAAE) to prevent ischemic stroke or systemic embolism in patients without AF who are undergoing cardiac surgery.
Overall, as it stands in the 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation, surgical exclusion of the LAA can be considered in patients with AF already undergoing cardiac surgery, as determined by the patient’s heart team (Class IIb, Level of Evidence B- non-randomized, upgraded from C).
Percutaneous LAA occlusion
The primary downside of surgical LAA occlusion is that it holds minimal appeal as a stand-alone procedure. The trials investigating its utility included only patients undergoing cardiac surgery for another indication. Percutaneous means to occlude the LAA are intuitively attractive as they may be equally (or more) effective with substantially less physiologic insult to the patient and an overall improved safety profile compared to surgical closure or even medical therapy. A number of devices have thus far been developed for occlusion of the left atrial appendage. Initial devices included the Percutaneous Left Atrial Appendage Occluder (PLAATO, eV3, Inc., Plymouth, MA, USA) and the Watchman Left Atrial Appendage System (Boston Scientific Corporation, Marlborough MA, USA). Additionally, Amplatzer ASD and VSD devices (St. Jude Medical, St. Paul, MN, USA), although originally not intended to occlude the left atrial appendage, have been utilized to occlude the LAA. The Amplatzer Amulet left atrial appendage occluder is an iterative design of the ACP, specifically developed for atrial appendage occlusion, and it has recently become available in a number of countries. Additionally, the WaveCrest (Coherex Medical, Salt Lake City, UT, USA), Ultraseal (Cardia Inc, Eagan, MN, USA) and LAmbre (LifeTech Shenzhen, China) devices are three newer devices with CE mark approval. Finally, the Lariat device (SentreHEART, Redwood City, CA) provides an extracardiac means to ligate/occlude the left atrial appendage.
Transcatheter Anatomical Considerations
The interatrial septum is important to examine for the presence of patent foramen ovale (PFO), thickened septum, atrial septal aneurysm, or prior patch repair. The presence of these can help to determine the feasibility of device closure along with the selection of type and size of the LAA closure device. Trials involving LAAO device placement generally exclude patients with congenital heart disease such as atrial septal defect or septal aneurysms.
Knowledge of the LAA’s diameter and depth is key when selecting the dimensions of the device. One of two core device-sizing selection principles to strive for is device-diameter matching and adequate device compression, defined as achieving 8-20% of device compression by the LAA ostium. This is accomplished by choosing the largest device as allowed by the LAA depth that simultaneously reaches ideal compression, without oversizing the device (not exceeding device compression of greater than 30-35% of the maximal ostial diameter). The spectrum of existing ostial shapes should be accounted for when measuring maximal ostial diameter. Available devices are generally round whereas most ostia are oval; thus, device selection to maximal diameter aims to prevent incomplete sealing and peri-device leak. The second principle in device-sizing selection is to measure LAA depth, defined as the midpoint of the diameter line of the maximal ostial orifice to the most distal point in the LAA, usually at the apex of the most anteriorly-directed lobe. If there is an instance where the maximal depth of the LAA is shallower than the shortest device that is wide enough to allow good compression, then that device may not be used. As the LAA consists of relatively thin, friable tissue, overly deep device placement may lead to LAA rupture, hemopericardium, and tamponade. Failure to circumnavigate the given LAA anatomy can lead to the need for intraprocedural resizing, potential complications, and longer procedure times.
Preprocedural Imaging
Intraprocedural transesophageal echocardiography (TEE) and fluoroscopy remain the established standard imaging modalities for assessment of anatomic variation, device positioning, assessment of successful deployment, and monitoring of complications during percutaneous transcatheter LAAO device delivery (Figure 2 and Figure 8). Preprocedural imaging with three-dimensional computed tomography (CT) or magnetic resonance imaging (MRI) scan can be useful for device sizing selection and characterization of complex LAA anatomy. In fact, CT has been shown to be superior to TEE for LAA morphology characterization, accurate device sizing, and evaluation of surrounding extracardiac structures and comparable to TEE for evaluation of thrombus. In the single-center, Prospective, Randomized Comparison of Three-Dimensional CT Guidance Versus TEE Data for LAA Occlusion (PRO3DLAAO) trial, CT-guided LAAO case planning was associated with greater accuracy in device selection on the first attempt (92% versus 27% with 2D-TEE; p = 0.01), although with the allowance of intra-procedural upsizing in the 2D-TEE cohort, the 2D-TEE cohort accuracy increased to 64% (P = 0.33). This study found significantly increased efficiency during the LAA procedure with CT versus 2D-TEE; less devices and guide catheters were used and procedure time was reduced.
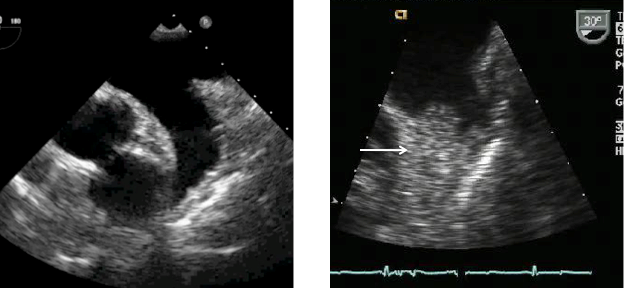
Figure 2
LAA appendage
Left: Transesophageal echocardiographic image of a normal left atrial appendage.
Right: Left atrial appendage with significant thrombus (arrow)
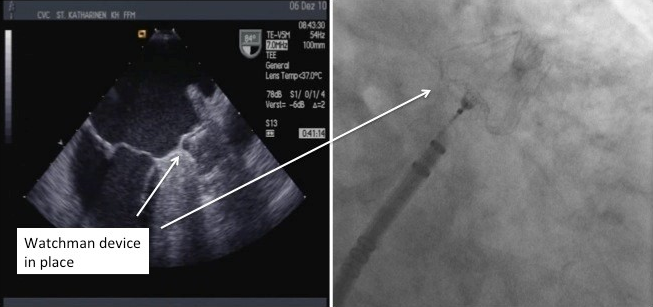
Figure 8
Fluoroscopic and Echocardiographic view of deployed Watchman device.
Transseptal Puncture (TSP) Technique
TSP was first utilized in 1959 for hemodynamic measurement of LA pressure with a chief goal of crossing the interatrial septum (IAS) mainly via the fossa ovalis (FO). Since the advent of structural and electrophysiologic interventions, TSP has been used with more attention to site-specific access. The ideal TSP site for LAAO differs in comparison with other structural interventional device TSP sites, depending on the anatomic needs of the landing zone for the device. When performing LAAO, it is important to remember the length of the LAA is directed anteriorly, and the central axis of the LAA ostium lies perpendicular to the body of the LAA. Landing the LAAO device successfully in the ostium depends on positioning the delivery sheath with enough depth within the LAA. With the anterior orientation of the LAA, a posterior-anterior trajectory of the sheath is most suitable for maneuverability within the LAA. Starting from a posterior TSP provides the most favorable sheath orientation and flexibility for directing the sheath anteriorly. Starting from a position that is too superior or anterior will complicate sheath alignment through the ostium into the long axis of the LAA. Additionally, a posterior position avoids inadvertent puncture of the aorta. Of note, different LAAO devices require slight modifications in TSP height, depending on the shapes and trajectories of the delivery sheaths. For example, the Watchman and Amplatzer Cardiac Plug are best delivered from a mid-septal to slightly inferior TSP, whereas the LARIAT device is better delivered slightly more superiorly.
Traditionally, TEE guides intraprocedural TSP. The bicaval view (100 to 120 degrees) is the primary working view, which displays the superior-inferior axis of the atrial septum. Biplane mode on TEE allows simultaneous visualization of the secondary working view, the orthogonal short-axis view (10 to 30 degrees), which displays the anterior-posterior axis of the atrial septum. For purposes of orientation, the bicaval view shows the superior-inferior axis with superior to the right (toward the SVC) and inferior to the left (away from the SVC); the short-axis view shows the anterior-posterior dimension with anterior to the right (toward the aortic valve [AV]) and posterior to the left (away from the AV). Clockwise rotations move the apparatus more posteriorly, whereas counterclockwise rotations move the apparatus more anteriorly. Intracardiac echocardiography (ICE) is an alternative to TEE for intraprocedural TSP guidance and it has several advantages that may make it preferable for some operators. Procedures may be more efficient as the primary operator can manipulate the ICE probe instead of depending on anesthesia for TEE guidance and reduce the necessity of intubation. The small caliber of the catheter (8- to 10-F) minimizes the cluttered appearance on fluoroscopic views. However, ICE requires additional vascular access.
Crossing the IAS for TSP is best achieved under echocardiographic visualization. There are numerous devices for crossing, employing needle, wire or radiofrequency to aid in successful puncture. Upon crossing the septum, heparin is given for a goal activated clotting time of 250 seconds, and the dilator is advanced. If this encounters resistance, the atriotomy site can be modified with coronary balloon dilatation or balloon-assisted crossing. Sheath-uncrossable cases should be managed similarly via additional dilation of the IAS.
Once transseptal access is completed, a sheath is placed across the septum and a pigtail catheter is inserted into the LAA. This technique minimizes trauma to the LAA on engagement. Multiple fluoroscopic and echocardiographic measurements are made to accurately define the diameter, angle and depth and specific anatomy of the atrial appendage (Figure 3, Figure 4 and Figure 5). After the appropriate size is determined, the device is positioned in the LAA, and the delivery sheath is retracted, thus deploying the device. Upon determination of successful deployment, the delivery sheath is detached from the device. At this point, the sheath is withdrawn into the right atrium. Details pertaining to each individual device are discussed below.
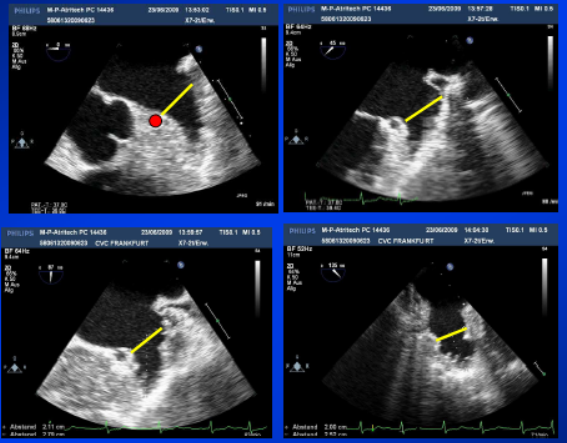
Figure 3
Echocardiographic measurements of the Left Atrial Appendage
Echocardiographic measurements of the left atrial appendage. Multiple views are taken to optimally visualize the LAA landing zone width.
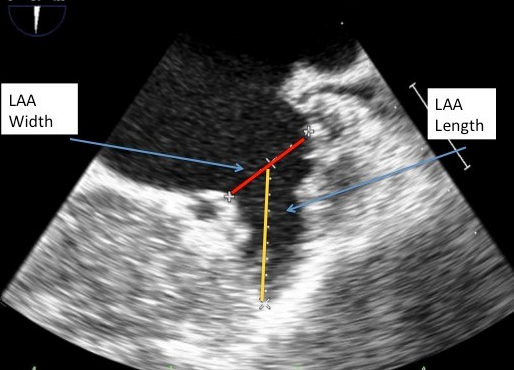
Figure 4
Echocardiographic measurements of the LAA length and width.
Echocardiographic measurements of LAA length (yellow) and width (red) taken in 45 degree view of the LAA.
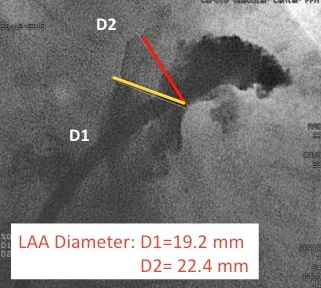
Figure 5
Angiographic measurments of the LAA.
Angiographic measurements (RAO caudal) of the LAA.
Combined Percutaneous Procedures
As in the surgical sphere, where LAAE is being done simultaneously with other planned cardiac procedures due to safety and convenience, there is also an increase in popularity of tandem LAAO and other procedures, such as AF ablation. Between 2016 and 2019, there was a 60% increase in combined AF ablation and LAAO procedures. When compared with standalone matched LAAO or AF ablation procedures, there was no significant difference in major adverse cardiac events (MACE) or 30-day readmission rates with combined AF ablation and LAAO procedures. The WATCH-TAVR trial is currently underway to compare all-cause mortality, stroke, and bleeding rates in patients with AF who are undergoing transcatheter aortic valve replacement (TAVR) and LAAO with those undergoing TAVR alone.
Percutaneous Devices
PLAATO
The Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO) device (Figure 6) was the first patented LAA occlusion device, designed by Dr. Michael Lesh and sponsored by the company Appriva. Dr. Horst Sievert was the first to successfully implant the PLAATO device in humans in 2001. LAAO devices were developed in the peak era of coronary stent development, so design concepts such as self-expansion carry over. The device consisted of a self-expandable balloon-shaped nitinol cage 15 to 32 mm in diameter and was coated with expanded polytetrafluoroethylene (PTFE). A membrane covered the atrial surface of the device, and the device anchored into the LAA ostium using three rows of projecting anchors. In 2005, Ostermayer et al., reported the results of a nonrandomized international multicenter prospective feasibility trial in 111 patients with non-rheumatic atrial fibrillation of at least 3 months duration who had contraindications to therapy with warfarin. The PLAATO system was successfully implanted in 97.3% of patients (108/111). Post-procedural antiplatelet therapy consisted of acetylic acid and clopidogrel. Four patients developed pericardial effusions or cardiac tamponade, and pericardiocentesis was necessary in three of them. One patient experienced a hemothorax and another a pleural effusion. At 6 months follow-up, successful LAA occlusion was demonstrated in 98% of the patients with a transesophageal echocardiogram. One patient developed a laminar thrombus on the device, detected at routine 6-month follow-up. During a follow-up period of 91 patient-years, two patients had a stroke, leading to an annual stroke rate of 2.2% after successful left atrial appendage occlusion. This represents a 65% relative risk reduction compared to a CHADS2 score predicted stroke rate of 6.3% in this patient cohort. Extending results out to 5-years, Block et al., reported on 64 patients entered into the North American cohort of this study. Over 239 patient years of follow-up, the annualized cerebral events rate in this population (mean CHADS2 score of 2.6) was 3.8% while the expected rate was 6.6%. While the results demonstrated feasibility and acceptable risk profiles, there were criticisms. LAAO devices are designed to prevent adverse events such as stroke, so any causative embolic or adverse event from device placement represents a prohibitive liability. The need and duration for antiplatelet therapy was not systematically addressed. Although the authors desired larger studies to expound on these questions, the company was unable to design and fund randomized controlled trials that would navigate the device to regulatory approval, and so this foundational prototype was discontinued from production and sale.

Figure 6
PLAATO device (EV3, Plymouth, MA, USA)
Watchman
Following successful implantation of the PLAATO device in 2001, the first Watchman device (Atritec Inc, Plymouth, MN) was implanted in 2002. The Watchman was developed by interventional cardiologists in conjunction with engineers at Atritec Inc, a medical startup company. The most important differentiating factor from a design standpoint for the Watchman, in contrast to the PLAATO device, was its open-end distal design as well as a semipermeable fabric covering rather than a closed-end design with PTFE covering (Figure 7). This design strategy proved able to accommodate a larger range of anatomies and heal within the endocardium more completely and quickly. An important early hurdle for the device was thrombus formation on the polymer covering observed in the early days after implantation, which was ameliorated in preclinical studies by adding warfarin therapy for 6 weeks post-implantation to allow time for device seal within the endocardium. The company worked with the FDA early on in its nascent clinical experience phase, building a portfolio of clinical studies which subsequently led to two successful randomized clinical trials (PROTECT AF and PREVAIL).
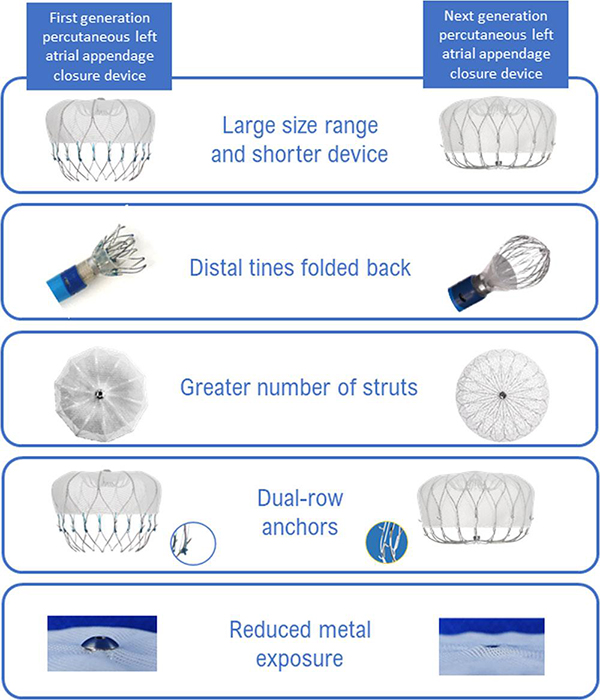
Figure 7
Watchman versus Watchman FLX devices.
Watchman FLX (Boston Scientific, Minneapolis, MN, USA) Reprinted with permission from Kar S, Doshi S, Sadhu D, et al. Primary Outcome Evaluation of a Next-Generation Left Atrial Appendage Closure Device. Circulation. 2021;143:1754–1762. DOI:10.1161/CIRCULATIONAHA.120.050117
In a formative 2007 safety and feasibility study of the early Watchman device, Sick et al., reported the initial experience of 75 patients. The device was successfully implanted in 66/75 (88%) of patients with 93% complete sealing of the LAA at 45 days. Device embolization occurred in two of the initial 16 patients with successful retrieval. This led to redesign of the fixation barbs with no further embolic instances with suggestion of improvement in stroke risk. Additional adverse events included cardiac tamponade in two patients, air embolism in one patient, and delivery wire fracture requiring surgery in one patient. Four patients developed a flat layer of thrombus on the device at 6 months, and these resolved with additional anticoagulation. Two patients experienced a transient ischemic attack (mean CHADS2 score of the 66 patients was 1.8 ± 1.1). (36) Fifty-five of 60 patients (91.7%) seen at 6-month follow-up had discontinued warfarin therapy. There were no cerebral events during a mean follow-up of 24 months.
In the 2009 PROTECT AF (Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority) trial, 707 patients with atrial fibrillation and a CHADS2 score of ≥ 1 were randomized to LAA occlusion with the Watchman device and planned discontinuation of warfarin after 45 days versus continued therapy with warfarin. Randomization was 2:1 favoring LAA occlusion. The study had a non-inferiority design with a composite primary efficacy endpoint of cerebral and systemic embolic events, and cardiovascular death. The primary safety endpoint was a composite of hemorrhagic events, pericardial effusion and device embolization. The device was successfully implanted in 88% (408/463) of patients, and at 45 days 86% of these patients were able to discontinue warfarin. Safety events were higher in the intervention group and peri-procedurally correlated. There was a higher rate of ischemic events in the intervention group, mainly linked to periprocedural air emboli. Three of five patients with ischemic stroke had no long-term deficits, whereas the remaining two were discharged to nursing homes and subsequently died. There was a 4.8% event rate of non-disabling, non-fatal pericardial effusions requiring drainage versus no events in the warfarin group. Events in the warfarin group occurred at any point along the timeline of duration of treatment. Hemorrhagic strokes were more frequent in the warfarin group. Five of six hemorrhagic strokes in the warfarin group were fatal. There was one fatal hemorrhagic stroke in the intervention group during the 45-day period on warfarin. Numerically, the rate of all strokes was lower in the intervention group but this did not reach statistical significance for superiority.
Cumulatively, mortality rates were 3.0% for the intervention group (95% CI 1.3–4.6) versus 3.1% for the control group (95% CI 0.8–5.4) at 1 year, and 5.9% (95% CI 2.8–8.9) versus 9.1% (95% CI 4.2–14.1) at 2 years.
The results of this trial have since been extended out to 2.3 years with a favorable impact on quality of life. Continued efficacy of the Watchman device was demonstrated at 4-year follow-up. In the 463 Watchman-treated patients compared to the 244 medically-treated patients, the primary endpoint was noninferior (2.3% vs. 3.8%, relative risk 0.60, 95% CI 0.41-1.05). Interestingly, at last follow-up all-cause mortality (3.2% vs. 4.8%, relative risk 0.66, 95% CI 0.45-0.98, p = 0.0379) and cardiovascular mortality (1.0% vs. 2.4%; relative risk 0.40, 95% CI 0.23-0.82, p= 0.0045) were lower in Watchman treated patients.
In view of lingering concerns from the United States Food and Drug Administration regarding the safety of the device after the results of the PROTECT AF trial, the 2014 Prospective Randomized Evaluation of the Watchman LAA Closure Device In Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy (PREVAIL) trial was designed to further explore the safety and efficacy of the device compared with standard-of-care warfarin. In the trial, 407 patients with nonvalvular atrial fibrillation and a mean CHADS2 score of 2.6±1.0 (a higher-risk group than in PROTECT AF) were randomized to either LAA occlusion with the Watchman device (n=269) or medical therapy (n=138) with a primary combined endpoint of stroke, systemic embolism and cardiac/unexplained death. At 18-months, the primary endpoint rate was 0.064 in the device group versus 0.063 in the control group (RR 1.07, 95% CI 0.57 to 1.89), and this did not meet the prespecified margin to satisfy noninferiority. The control group had unexpectedly low rates of events as compared to event rates found in other contemporary trials utilizing warfarin as stroke prevention in atrial fibrillation i.e., RELY and ARISTOTLE. The second noninferiority endpoint of stroke and system embolism >7 days post randomization was satisfied. On balance, this trial was limited by extremely low event rates in both groups, and the rates of pericardial effusion requiring surgical repair decreased in this trial to 0.4% (compared to 1.6% in PROTECT AF). Strokes caused by the procedure or related to the device decreased in PREVAIL (0.4%) as compared with PROTECT AF (1.1%, p = 0.007) These findings led to the general conclusion that LAA occlusion is both safe and effective.
While the Watchman had already secured Conformité Européenne (CE)-mark approval in 2005, the U.S. Food and Drug Administration only approved the Watchman device in 2015 after the PREVAIL and PROTECT AF results, to be used for patients at increased risk of stroke from non-valvular atrial fibrillation who qualify for warfarin but have a reason for pursuing mechanical anti-embolic methods after patient-physician discussion. Prior to this governmental imprimatur of the Watchman, the Continued Access to PROTECT AF (CAP) and the CAP2 Registries were established to provide long-term data on the safety and efficacy of the device. The CAP registry was nonrandomized and included 460 patients from 26 centers and included the same criteria and procedural protocol as described in PROTECT AF. In combination with data from PROTECT AF, the rates of serious pericardial effusion and strokes decreased between PROTECT AF and CAP. Effusion rates fell from 27 of 542 (5.0%) in PROTECT AF to 10 of 460 in CAP (2.2%; P=0.019), and stroke rates dropped from 5 of 542 (0.9%) to 0 of 460 (0%; P=0.039), indicating continued improvements in peri-procedural safety. The CAP2 registry of 578 patients supplemented the PREVAIL trial, with similar inclusion/exclusion criteria and protocol detailing. Patients in both CAP and CAP2 were at higher stroke risk with extensive comorbidities, yet had excellent procedural implantation success with lower complication rates. The lowest reported hemorrhagic stroke event rate was seen in the CAP registries (0.17 events per 100 patient-years in CAP and 0.09 events per 100 patient-years in CAP2), with low ischemic stroke event rates as well (1.30 events per 100 patient-years in CAP and 2.20 events per 100 patient-years in CAP2). Overall, the CAP/CAP2 registry data contribute more evidence that over time, the peri-procedural safety of the Watchman procedure is improving, though the data on efficacy in comparison to anticoagulation remains to be corroborated through further future randomized controlled trials.
A patient level meta-analysis evaluating bleeding outcomes for the 1,114 patients enrolled in PROTECT AF and PREVAIL over a median 3.1 years of follow-up was published by Price and colleagues. In this analysis, there were similar overall bleeding rates between patients treated with the Watchman device compared to those treated with medical therapy (3.5 vs. 3.6 events per 100 patient years, RR 0.95, 95% CI 0.66-1.40, p=0.84). However, when considering events occurring > 7 days post- randomization, there were significantly less events in the Watchman patients (1.8 vs. 3.6 events per 100 patient years, RR 0.49, 95% CI 0.32 – 0.75, p=0.001) with an even more significant difference noted after 6 months. This study demonstrates that the initial procedural risk is balanced by long-term benefit with regard to bleeding. In another meta-analysis of longer-term follow-up of both PROTECT AF and PREVAIL, while there was a trend toward a higher ischemic stroke rate in the Watchman group (p=0.08), the all-cause mortality was lower in the Watchman group (p=0.04) likely driven by the higher hemorrhagic and disabling/fatal stroke rate in the control group (p=0.0022 and p=0.04, respectively).
Currently, the Watchman FLX device is the latest, second-generation iteration of the Watchman device that consists of a self-expanding nitinol frame covered by a 160 µm polyester membrane on its atrial-facing side. Eighteen fixation barbs (versus 10 in the original Watchman) around the mid-perimeter secure the occluder to the wall of the left atrial appendage, reducing peri-device leak. The device is available in diameters ranging from 20–35 mm. Unlike earlier generations, Watchman FLX device has enfolded distal tines, dual-row anchors, more numerous struts, and reduced exposed metal (Figure 7). It has full recapture and repositioning capabilities, with multiple recaptures allowed. The enfolded tines also allow for the partially deployed device to form a “flexball” with a rounded end that can allow for atraumatic advancement to gain depth in the LAA during implant.
The safety and efficacy of the Watchman FLX device was evaluated in the PINNACLE FLX study (Protection Against Embolism for Nonvalvular AF Patients: Investigational Device Evaluation of the Watchman FLX LAA Closure Technology). This prospective, nonrandomized, multicenter study enrolled 400 patients with a mean age of 73.8 ± 8.6 years and a mean CHA2DS2-VASc score of 4.2±1.5. The primary safety endpoint was incidence of death, stroke, systemic embolism, or device- or procedure-related complications within 7 days of procedure or by hospital discharge (whichever occurred later). The efficacy endpoint was the presence of effective LAA closure defined as peri-device flow ≤ 5 mm at the 45-day and 12-month check-up. The performance goal of 4.2% was met for the primary safety endpoint, with a 0.5% incidence of safety events (2 patients, one ischemic stroke, one transient ischemic attack) (p < 0.0001). The performance goal of 97.0% was met for the primary effectiveness endpoint, with a 100% achievement of effective device closure (p < 0.0001). There were 7 patients with device-related thrombus; no patients experienced pericardial effusion requiring surgical drainage or device embolization. At a 2-year follow-up of 395 implanted patients, the secondary end point of 2-year stroke or systemic embolism was 3.4%, with an annual rate of 1.7%, surpassing the prespecified performance goal of 8.7%.
The overall results of PROTECT AF and PREVAIL studies suggest that LAA occlusion with the Watchman device is a reasonable alternative to warfarin therapy in patients with atrial fibrillation and elevated risk of cerebral events. One unknown that has been brought to light from these studies that has not been explored in a published, randomized trial to date is the role of LAAO in patients with contraindications to warfarin therapy. This question was delved into in the multicenter, prospective, nonrandomized ASA Plavix Feasibility with Watchman Left Atrial Appendage Closure Technology trial, in which patients received 6 months of dual-antiplatelet therapy followed by lifelong aspirin and no warfarin after Watchman placement. In this study, 150 patients with nonvalvular atrial fibrillation and a CHADS2 score of 2.8 ± 1.2 and a contraindication to oral anticoagulation (93% with history of hemorrhage or other bleeding tendency) were enrolled and followed for a mean 14.4 months post procedure. There were 6 cases noted of device-related thrombus (DRT), of which one was noted as a serious adverse event because it was associated with a stroke occurrence nearly a year post-implant. There were 4 patients with DRT that received 4-8 weeks of low molecular weight heparin, and the fifth case was managed without medication with no adverse event. Compared to an expected rate for ischemic stroke of 7.3% per year according to the CHADS2 score, patients undergoing LAA occlusion with the Watchman device had ischemic stroke rates of 1.7% per year. One patient suffered a hemorrhagic stroke (0.6% stroke rate per year). Adverse events occurred in 8.7% of patients and included bleeding, pericardial effusion and device thrombus/embolization. Overall, these results suggested that the Watchman device can be safely and effectively implanted without a warfarin bridge in patients with contraindications to warfarin therapy.
The EWOLUTION (Evaluating Real-Life Clinical Outcomes in Atrial Fibrillation Patients Receiving the Watchman Left Atrial Appendage Closure Technology) is another prospective, non-randomized, multicenter registry that examined the question of whether Watchman implantation was safe in patients with relative contraindications to anticoagulation (72%). The 1,020 patients from 47 centers were older with multiple comorbidities placing them at high risks for stroke and bleeding. There was a wide distribution in the postimplantation antithrombotic strategy used, with 60% receiving DAPT, 16% VKA, 11% NOACs, 7% antiplatelet monotherapy, and 6% receiving no treatment. During a median follow-up of 2 years, the stroke rate was 1.3% per year, with a combined end point of ischemic stroke, TIA, and systemic embolism rate of 2% per year, and 83% and 80% reduction in expected event rates in the untreated population. Furthermore, these rates are similar to the annual ischemic stroke rate (1.6%) and combined ischemic stroke, TIA, and systemic embolism rate (1.7%) from the combined data of PROTECT AF and PREVAIL, in which standard antithrombotic regimens were used. The rate of DRT in EWOLUTION, with its variable antithrombotic strategies, was 4.1%, whereas in comparison, the DRT rate in the combined analysis of PROTECT AF, CAP, PREVAIL, and CAP-2 was 3.7%. Overall, EWOLUTION showed consistent low stroke rates despite deviation from the traditional antithrombotic regimen, though this trial was limited by its lack of comparison group.
Designed to fill the knowledge gap of whether LAAO device placement is safe and efficacious in high-stroke risk (CHA2DS2-VASc score ≥ 2) patients ineligible for warfarin, The Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation (ASAP-TOO) trial is an ongoing prospective, randomized, multi-center, global trial enrolling up to 888 patients randomized in a 2:1 ratio of Watchman versus control. Control patients will receive antiplatelet monotherapy or no therapy. The anticipated results release is in December 2023. The primary safety endpoint encompasses the 7-day combined rate of all-cause death, cardiovascular accident, systemic embolism and periprocedural complication requiring surgery or endovascular intervention. The primary efficacy endpoint is measured as time to first ischemic event or systemic embolic event. Secondary outcome endpoints are the occurrence of cardiovascular death, ischemic and hemorrhagic stroke, systemic embolism, and major bleeding.
In September 2022, the FDA approved DAPT usage for the Watchman FLX in light of data from prior trials and the LAAO Registry. Recent data discussed at Transcatheter Valve Therapy (TVT) 2022 comparing DAPT with aspirin plus an anticoagulant or warfarin after Watchman implantation showed no difference in deaths, strokes, major bleeding episodes, or DRT. Interestingly, DAPT has been an option after Watchman device placement since 2017 in European countries.
While most randomized trials to date have examined Watchman device placement in patients who are eligible for warfarin yet have reasons for pursuing mechanical antithrombotic treatments, the CHAMPION-AF clinical trial is randomizing approximately 3000 patients with atrial fibrillation to either the Watchman FLX or a NOAC in a direct comparison of the two strategies. Warfarin has largely fallen to the wayside as an anticoagulation comparator in trials due to its higher incidence of bleeding, including intracranial hemorrhage. In CHAMPION-AF, patients who are perfectly eligible for a NOAC but prefer an LAAO device will be compared with those who opt to take NOACs. The primary outcome measures are measuring noninferiority of the WATCHMAN FLX for occurrence of stroke, cardiovascular death, and systemic embolism, and superiority in regards to non-procedural bleeding. Results are expected in 2027. The only randomized controlled trial thus far to directly compare NOAC with LAAO (Watchman or Amulet) has been the PRAGUE-17 (Left Atrial Appendage Closure vs Novel Anticoagulation Agents in Atrial Fibrillation) trial. In this noninferiority trial, the primary endpoints were a composite of stroke, death, bleeding, and procedure-related complications and ultimately showed that at 20 months LAAO was noninferior to NOAC (95% apixaban). Because the trial was not powered to detect differences between the groups in the individual components of the primary endpoint, and because the benefits with regards to LAAO are seen in the long-term, the PRAGUE-17 authors conducted a 4-year follow-up of 402 patients. Again, there were no significant differences in long-term follow-up for all-stroke, TIA, cardiovascular mortality, or clinically relevant bleeding. However, when excluding bleeding events that occurred procedurally, the nonprocedural bleeding events were significantly reduced in the LAAO group (p = 0.049). In fact, a look back at a temporal analysis of bleeding events in PRAGUE-17 shows a separation in curves in favor of LAAO as early as 6 months. On balance, not only does this finding reinforce the commonly cited issues with long term anticoagulation, it further emphasizes the premium on procedural safety during LAAO to assure optimal long-term patient outcomes. Because PRAGUE-17 is underpowered, it is difficult to draw conclusions for the individual endpoints such as stroke rate. The projected higher power of CHAMPION-AF should shed further light on the question of the individual risks and benefits of LAAO versus NOAC.
Amplatzer devices
First use of the Amplatzer Septal Occluder (ASO) began in 2002, around the same time as the Watchman. Originally designed for atrial septal defect closure with a double disk mechanism (one disk for the left atrial side and one for the right atrial side), the ASO was serendipitously discovered to fit into the LAA when the left disk was accidentally deployed in the LAA and then became difficult to remove. With knowledge of the original difficulties of the PLAATO device, it was thought that ASO placement would be a much simpler technique given its success for ASD closure. Thus, the ASO was regeared for LAAO, and the Bern technique was developed based on the pacifier principle. Reminiscent of a pacifier, the final orientation of the ASO using the Bern technique is such that the left-sided disk sits lodged in the LAA and the right-sided disk faces the atrial chamber. Meier et al., reported on the first 16 patients who underwent LAA occlusion with this device. Implantation was successful in 15 patients with one patient experiencing device embolization (device larger than appendage) with uneventful surgical removal and right/left appendage obliteration. During follow-up, complete occlusion was noted in all remaining 15 patients with no further complicating events. However, in 100 patients, the acute embolization rate was 10% so the technique was abandoned and the device was redesigned for tailored LAAO usage.
The Amplatzer Cardiac Plug (ACP) (Figure 9 a) was the brainchild of the reengineering efforts, receiving CE mark approval in 2008. Designed as a double-seal structure, it consists of a distal lobe with a proximal disk connected by a flexible central waist. Procedurally, the distal lobe is positioned within the left atrial appendage and the proximal disc is angled to fully cover the orifice of the left atrial appendage. Six pairs of hooks are attached to the distal body enabling the occluder to engage the wall of the left atrial appendage. The device is fully repositionable and recapturable. Device sizing is with reference to the distal lobe and ranges from 16-30 mm in 2 mm increments. The proximal disks are 4 mm larger for lobe sizes 16-22 mm and 6 mm larger for lobe sizes 24-30 mm. Appropriate sizing is 10-20% larger than the minimum appendage orifice measurement by TEE.
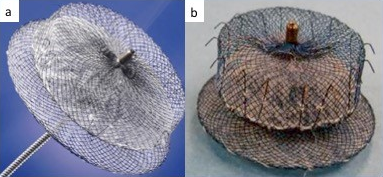
Figure 9
Amplatzer Cardiac Plug (left) and Amulet Device (right)
The initial European experience with ACP was reported for 143 European patients with atrial fibrillation. Device implantation was successful in 132/137 (96%) attempted implants. Serious adverse events occurred within 24 hours in 10 (7%) of patients. These events included 3 cerebral events, 2 device embolizations and 5 pericardial effusions requiring drainage. Subsequently, Kefer et al., reported their experience of 60 patients with atrial fibrillation (CHADS2VASc 4.4±1.8, HAS-BLED 3.3±1.3) undergoing LAA appendage occlusion with the ACP device. The device was successfully implanted in 59 patients with 3 cases of tamponade, 3 smaller effusions, 2 cases of air embolism and 1 pseudoaneurysm. Over 1 year follow-up, the rate of stroke was 2.14%/yr. For patients with contraindications to anticoagulation, Wiebe et al., reported their experience with 60 patients (CHADS2VASc 4.3±1.7, HAS-BLED 3.3±1.0) undergoing LAA occlusion with the ACP device. The procedure was successful in all but 3 patients. Over a mean of 1.8 years follow-up (1.0-2.8), no patients experienced stroke. Most recently, Tzikas et al., reported experience with the first generation Amplatzer cardiac plug in 22 centers involving 1,047 patients. Procedure success rate was high at 97%, and overall periprocedural complications were low at 4.3% (death, tamponade, stroke, bleeding, myocardial infarction, and device failure). On follow up, the observed stroke rate was 2.3% versus an expected 5.62% based on CHADS2VASc score risk stratification with an overall reduction of 59%.
Amulet
The Amulet (Figure 9 b) is an iterative design advance on the original ACP device. Modifications of the Amulet include preloading for enhanced ease of use, availability of 31 and 34 mm sizes that allows for closure of a wider range of LAA diameters (11- 31 mm), a longer waist and greater number of fixation wires to enhance stability and a recessed pin to minimize thrombus formation. The first-in-man evaluation of this second-generation LAAO device included 25 patients with atrial fibrillation and a mean CHA2DS2VASc score of 4.3 +/- 1.7 undergoing LAA occlusion with the Amulet device. Procedural success was 96% with no procedural complications and complete closure in all patients. One patient experienced device-related thrombus with no clinical consequence. These findings led to CE approval in early 2013. A head-to-head comparison of the Amulet and ACP devices was performed in 59 patients (31 ACP, 28 Amulet). There were no differences in procedural success or device related complications, but less patients in the Amulet group had any form of residual leak on follow-up TEE (8% Amulet vs. 48% ACP, p=0.01).
Because the LAA has a wide range of morphologies, it was hypothesized that the dual-seal mechanism of the Amulet may be more robust in closing the appendage than the single-seal mechanism of the Watchman. In order to provide a head-to-head comparison of the safety and effectiveness of the first-generation single-seal Watchman with the second-generation double-seal Amulet, the recent Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet Investigational Device Exemption (IDE)) trial was completed and published in 2021 as the first large-scale, multicenter, randomized-controlled trial of 1878 patients, paving the way for U.S. FDA approval of the device in August 2021. The Amulet was found to be noninferior to the Watchman for the primary safety endpoint (a composite of procedure-related complications, all-cause mortality, or major bleeding at one year) ((14.5% versus 14.7%; difference=–0.14 [95% CI, –3.42 to3.13]; P<0.001 for noninferiority). Additionally, it was noninferior to the Watchman device for the primary effectiveness endpoint (composite rate of cerebral vascular accident, systemic embolism, or cardiovascular death (5.6% versus 7.7%, difference=–2.12 [95% CI, –4.45 to0.21]; P<0.001 for noninferiority) and the composite of rate of ischemic stroke or systemic embolism (2.8% versus 2.8%; difference=0.00 [95% CI, –1.55 to1.55]; P<0.001 for noninferiority).
Effectiveness was also measured as successful left atrial appendage occlusion with residual jet ≤5 mm at 45 days on TEE, with 98.9% of patients with the Amulet meeting this threshold compared with 96.8% of Watchman patients (difference, 2.03 [95%CI], 0.41–3.66]; P<0.001 for noninferiority, P=0.003 for superiority). Total occlusion with no residual jet was found in 63.0% of Amulet patients and 46.1% of Watchman patients. There were higher rates of procedural complications (pericardial effusion and device embolization) for the Amulet versus Watchman (4.5% versus 2.5%), which decreased with operator experience. Physicians outside the United States were more familiar with the implantation process of Amulet, and American physicians were provided an opportunity prior to the trial to implant up to 3 Amulets to gain experience. Rates of major bleeding were similar between Amulet (11.6%) and Watchman (12.3%) (P=0.32) and thus the secondary endpoint of superiority was not met.
In an analysis particularly focusing on the peri-device leak incidence and outcomes in the Amulet IDE trial, Price et al., redefined clinically significant PDL as ≥ 3 mm detected at 45 days and 12 months post-procedure. Given the risk of PDL as a source for thrombus formation and subsequent systemic embolism, the primary endpoint was chosen as ischemic stroke or systemic embolism, with the secondary endpoint being a composite of stroke, systemic embolism, or cardiovascular death. The Amulet was superior with regards to closure (leak <3 mm) compared with Watchman both at 45 days (88.9% vs 74.1%; P < 0.01) and 12 months (90.5% vs. 78.3%; P < 0.01). PDL was associated with a higher, albeit statistically insignificant, risk for stroke or systemic embolism at 18 months (3.6% vs 1.8%; adjusted HR: 1.98; 95% CI: 0.93-4.19; P = 0.07) but a higher, statistically significantly risk for the secondary endpoint (8.1% vs. 4.7%; adjusted HR: 1.66; 95% CI: 1.02-2.69; P = 0.04), emphasizing that appropriate seal is an important treatment goal for LAAO device deployment. One major limitation of the Amulet IDE trial is that the Watchman 2.5 was used as a comparator instead of the improved Watchman FLX version, which contains modifications intended to improve appendage occlusion. To compare the Amulet versus Watchman FLX, the Amulet or Watchman Device for Percutaneous Left Atrial Appendage Closure: Primary Results of the SWISS-APERO Randomized Clinical Trial was conducted at 8 European centers. Watchman FLX was implanted in 77% of the patients in the Watchman arm. Amulet did not achieve a lower rate of residual LAA patency compared with Watchman on 45-day cardiac computed tomography angiography (CCTA) imaging. PDL rates were lower with Amulet, procedural complications were higher with Amulet mainly due to bleeding, and clinical outcomes between Amulet and Watchman were similar. A meta-analysis comparing Amulet with Watchman FLX showed similar rates of PDLs >5 mm (0.34% vs 0.01%, P = 0.06); smaller leaks were not analyzed in this study.
An ongoing multicenter, prospective, randomized, clinical trial Prevention of Stroke by Left Atrial Appendage Closure in Atrial Fibrillation Patients After Intracerebral Hemorrhage (STROKECLOSE), with results due in 2028, will compare LAAO with the Amplatzer Amulet device against medical therapy in a 2:1 ratio in patients with intracerebral hemorrhage (ICH) within the past year and non-valvular atrial fibrillation. The primary outcome assessed are rates of ischemic and hemorrhagic stroke, systemic embolism, major bleeding, and death over 2 years. Secondary outcomes include early and late safety parameters. Approved by the FDA in 2020, another trial - the CATALYST trial - compares the Amulet with NOACs in 2650 patients at 120 sites in a 1:1 fashion, with results expected in April 2029. Results of these trials will provide further information on the safety of Amulet device implantation.
WaveCrest
The WaveCrest (Coherex Medical, Salt Lake City, UT, USA, acquired by Biosense Webster in 2015) is a next generation occlusion device (Figure 10 a) that received CE mark in Europe in 2013. The single-lobe nitinol device is designed to allow occlusion at the ostium with minimal device protrusion into the appendage (the device is short, therefore, appendage depth is rarely a limiting factor). The device works well for small LAA anatomies because of its fit into the proximal LAA neck, and does not even require a delivery sheath system. It has 20 retractable distal anchors distributed around the perimeter of the occluder designed to conform to irregular anatomy and maximize stability, and the anchors may be manipulated independently of the occluder. The PTFE outer coating is both occlusive and non-thrombogenic and a polyurethane foam faces the LAA. Additionally, the ability to inject distally allows for assessment of occlusion and stability by angiography. The device is available in 3 sizes: 22, 27 and 32 mm. In the initial trial of 73 patients, 68 (93%) had acute procedural success with 2 cases of pericardial effusion requiring drainage. At 45-day follow-up, successful closure (defined as less than a 3 mm residual leak) was present in 67 (92%) of patients. There were no cases of DRT or strokes.
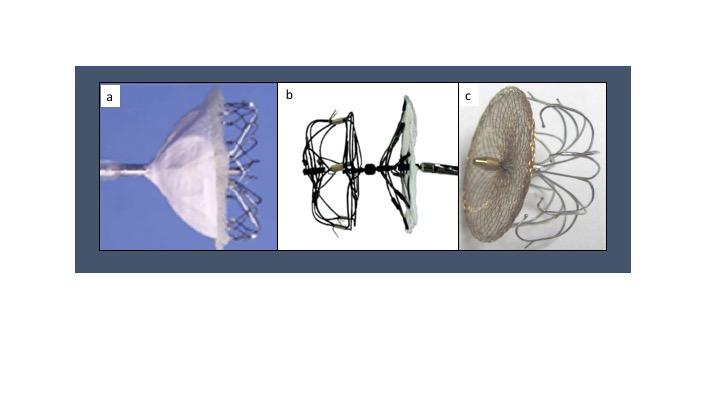
Figure 10
Additional Devices. WaveCrest (a), UltraSept (b), L’Ambre (c).
There are 2 ongoing studies to continue to expand on the safety and efficacy of the WaveCrest device. The first one is The WAVECREST Post-Market Clinical Follow-Up (PMCF) Study, a prospective, non-randomized, multicenter study in Europe to confirm the safety and efficacy of the WaveCrest in patients with non-valvular atrial fibrillation and higher risks of stroke, set to release results in 2023. Mortality and procedure-related complications, in addition to device deployment success, will be examined. The second trial, WAVECREST 2, is a prospective, multicenter, randomized trial comparing the WaveCrest with the Watchman in a 1:1 fashion in 1550 patients. Estimated to be completed in 2029, the trial is orchestrated to show that the WaveCrest’s safety and efficacy are non-inferior to the first-generation Watchman.
Ultraseal
With its design inspired by the Amplatzer Amulet’s “pacifier principle,” the Ultraseal device (Cardia Inc, Eagan, MN, USA) is another next generation device (Figure 10 b) with CE mark obtained in Europe in 2016. The device consists of an inner bulb with 12 stabilizing hooks and outer sail connected by a dual-articulating joint that simultaneously minimizes tension on the system and allows multidirectional movement to fit in complex LAA anatomies. There are 9 different bulb sizes available ranging from 16-32 mm (the 3-leaflet outer occluding “sail” is 6 mm larger than the bulb, with the diameter spectrum ranging from 22 to 38 mm). It is delivered through either a 10 or 12 French sheath, and is completely repositionable and retrievable.
In the initial feasibility and safety study of the Ultraseal, 23 patients underwent device placement at two Italian institutions after physician review of the LAA morphology. Procedural success was achieved in 21 patients; two of the patients (9%) experienced incorrect device deployment with partial LAA closure, which is a lower rate of incomplete closure compared with other devices in both randomized controlled trials and in large registries. At 45 days, TEE revealed no DRT on any device. PDLs were noted in the 2 patients whose devices were incompletely deployed; the first patient had a small PDL of 2-3 mm and the second patient had a moderate PDL of 5 mm.
Because of concerns of device fracture in a series of the first Ultraseal device, a second-generation Ultraseal was developed, with reduced length of the articulating joint, modification of the sail seal, addition of radiopaque markers, and removal of the central post on the inner bulb. In the LIGATE study, or Left atrial appendage closure with the II generation Ultraseal device: An international registry, 52 patients in 8 European centers were implanted. All patients had successful device deployment and correct implantation location, and technical success was achieved in all but 2 of those patients. One patient’s device perforated and the other had a severe (>5 mm) leak. A minor periprocedural stroke occurred in the patient with severe leak. There were no device fractures, DRTs, strokes, systemic emboli, or transient ischemic attacks during follow‐up. Three patients died post-procedurally of causes unrelated to device placement. Pivato et al., concluded high procedural success with low complication rates seen in LIGATE, however, they would need larger and longer studies to further conclude on safety and efficacy.
LAmbre
The LAmbre device (LifeTech Scientific Co. Ltd., Shenzhen, China) (Figure 10 c) received its CE approval in 2016, the same year as the Ultraseal. This device is a self-expanding dual membrane design (hook-embedded umbrella and fabric cover) connected by a short, central waist. It is available in two configurations, depending on the LAA size and lobation; the standard version occludes single-lobed LAAs and the special design occludes multi-lobulated LAAs. Seventeen different combinations are available with sizes ranging from 16-36 mm for the umbrella and 22-40 mm for the cover. The occluder is delivered through an 8-10 French sheath depending on the particular size chosen. The implantation technique is unique compared with other device deployments. The delivery sheath is placed at the ostial LAA and to deploy the umbrella, the delivery cable is pushed forward. Unsheathing releases the cover over the LAA ostium. The LAmbre comes with full recapture and redeploy capabilities. The CE mark study was based on a 60 patient experience demonstrating 100% success with complete closure in all cases and a 3.3% adverse event rate (1 patient died secondary to LAA wire perforation and another developed a pericardial effusion). A prospective, multicenter study in China of the LAmbre implantation experience in 153 patients demonstrated successful LAA occlusion in 152 patients. There were serious complications in 5 patients. During the 12-month follow-up, clinically significant pericardial effusion requiring intervention was detected in 3 patients, ischemic stroke occurred in 2 patients, incomplete LAA sealing occurred in 1 patient, and there was no device embolization. The LAmbre has been compared with the Watchman and Amulet in several trials suggesting similar safety and efficacy profiles. Larger randomized trials are needed to further confirm these findings. In March 2022, LifeTech received FDA approval for its pre-market, prospective, randomized, controlled clinical trial enrolling up to 3,000 patients to evaluate the safety and efficacy of the LAmbre occluder versus oral anticoagulants. Further data is needed on the LAmbre before it becomes more widely used.
Lariat
While the aforementioned devices occlude the left atrial appendage via intracardiac device implantation, the Lariat device (SentreHEART, Redwood City, CA, Figure 11 and Figure 12) occludes the LAA via a dual endo-epicardial mechanism. Preprocedural computed tomography is used to probe the suitability of LAA anatomy for the LARIAT device (generally 60-80% of patients will have favorable anatomy). In the procedure, epicardial access is obtained via a subxiphoid approach and a 14F epicardial guide is inserted. Transseptal access is gained via standard techniques and the left atrium is visualized under cineangiography. A 15-mm balloon tipped catheter is advanced over a 0.025” magnet tipped guide wire to the left atrial appendage. Next, a 0.035” magnet tipped epicardial guide wire is advanced through the epicardial sheath to the appendage so that the two magnets with opposing polarity connect in an end-to-end manner. Once this is accomplished, the Lariat snare is advanced over the 0.35” epicardial guide wire into position around the LAA. A balloon tipped catheter may be advanced to assist in positioning over the ostium of the LAA. As the snare is closed, under TEE and fluoroscopic imaging, flow in the LAA is assessed. When fully occluded, the suture is closed, the snare is removed and a suture cutter is advanced. A pericardial drain is exchanged over the 0.035” guide wire and the remaining equipment is removed. The drain is removed as appropriate.
Figure 11
Lariat Device
Lariat Device (SentreHEART, Redwood City, CA): A and B. Suture mediated delivery system. C. Magnetic guide wire system. D. Balloon occlusion system.
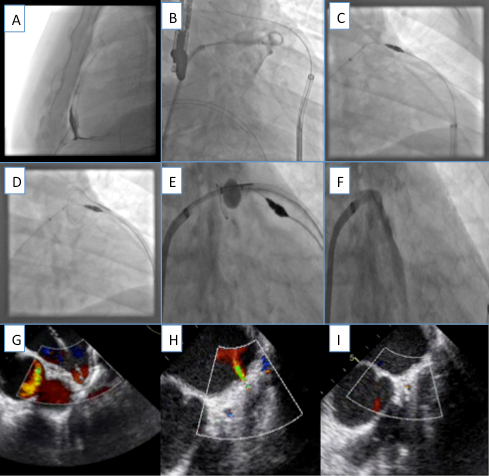
Figure 12
Lariat deployed
Deployment of Lariat device. A. Lateral view demonstrating epicardial access. B. Pigtail injection into LAA. C. Magnetic system in contact via endocardial and epicardial approaches. D. Suture delivery system advanced over epicardial guide wire. E. Balloon catheter at LAA ostium via endocardial approach. F. Suture deployed and contrast injection demonstrating complete occlusion of LAA. G. TEE view with endocardial device in place. H. Partial LAA occlusion. I. Complete LAA occlusion by TEE.
The Lariat device was evaluated in 89 patients with nonvalvular atrial fibrillation, a CHADS2 score ≥ 1 and contraindications to oral anticoagulation. Important exclusions included both clinical and anatomic features as demonstrated by preprocedural CT scan. Clinical exclusions consisted of history of pericarditis or cardiac surgery, pectus excavatum, prior thoracic radiation, ejection fraction less than 30%, myocardial infarction within 3 months, NYHA Class IV heart failure or thromboembolic event within 30 days. Anatomic exclusions included a LAA diameter > 40 mm, multilobed LAA in different planes exceeding 40 mm, a posteriorly rotated heart or a superiorly oriented LAA such that the apex extends behind the pulmonary trunk. Of the 89 patients treated, 85 were successfully closed with immediate complete occlusion in 81 with evidence of < 1 mm flow by TEE. Adverse events included late pericardial effusion (n=1), pericarditis (n=2), sudden death (n=2) and non-embolic stroke (n=2). At one-month follow-up, complete closure rates were 91%.
Subsequently, results from the retrospective U.S. transcatheter LAA ligation consortium were published (154 patients) that showed an unusually high major complication rate of 9.7% (primarily related to pericardial bleeding). In 2015, the FDA released a serious adverse effects warning for the Lariat device. That same year, the Lariat device won CE mark approval in Europe. In 2016, the device was improved to the LARIAT+ to contain a larger snare and enhanced maneuverability in order to reduce procedural complications. This device demonstrated a better safety profile than that reported in the aforementioned study (58 patients, no peri-procedural complications with the exception of one late pericardial effusion, complete closure rate of 92%, all leaks were <3 mm). The better safety profile was likely related to increasing experience with the system as well as with changes in pericardial access technique including the routine use of a micropuncture system. The same year the FDA issued a serious adverse events warning on the Lariat, it had also granted IDE approval for the Lariat to be investigated in the randomized, controlled AMAZE trial, designed to evaluate whether the Lariat device served as an adjunctive therapy to atrial fibrillation ablation in patients with persistent atrial fibrillation. In this prospective, multicenter, randomized trial of 610 patients, the primary safety endpoint of composite events detected 30-days post-Lariat compared to a 10% performance goal was 3.4%. Three patients experienced serious cardiac injury requiring surgical intervention, 8 patients experienced bleeding events requiring transfusion, and one patient sustained a vascular injury. The trial did not find superiority in combined LAA ligation and pulmonary vein isolation in preventing recurrent AF. Longer-term follow-up and randomized, controlled trials are necessary in order to establish the ultimate role of this device.
Emerging LAAO Devices
There are several emerging devices for LAAO that have either won CE-mark approval or are currently under clinical investigation. The Occlutech LAA Occluder (Occlutech International AB, Helsingborg, Sweden), CE-mark approved in 2016, has a polyurethane cover and a cone-shaped nitinol wire mesh. There is also the Transcatheter Sideris Patch (Custom Medical Devices, Athens, Greece); the Pfm device (Pfm Medical, Köln, Germany); the Sierra Ligation System (Aegis Medical Innovations, Vancouver, Canada) which is an epicardial LAAC device without the need for transseptal access; the dual-seal SeaLA LAA occluder; the Omega LAA occlusion device (Vascular Innovations, Thailand), a dual-layered “disc-and-cup” design; the Conformal LAA Seal (Conformal Medical), a self-expanding nitinol mesh covered with atraumatic foam and a PTFE cover; the Acoredis LAA occluder; the Prolipsis device; and novel occlusion devices such as the Laminar (Laminar) and Appligator (Append Medical) that use infolding of the LAA. , , , , The evaluation of these devices will continue to add to the safety and efficacy literature of LAAO and potentially offer more variety of choice to the structural toolkit of LAA closure.
Peri-Procedural Complications
Pericardial Effusion
Pericardial effusion (PE) is a rare complication post-LAAO implantation and a key monitored safety endpoint in randomized controlled trials involving LAAO devices. The mechanism for PE formation is via trauma to the thin-walled LAA after catheter and device manipulation on placement, followed by microtears from the anchors and outward radial force from the device. The National Cardiovascular Data Registry (NCDR) LAAO Registry is a prospective, national, audited registry consisting of 65,355 patients who received a Watchman (first-generation) procedure between January 1, 2016 and December 31, 2019. In this large registry, the incidence of PE requiring percutaneous drainage or surgical intervention was 1.35% (881 patients). Of those, 771 patients (1.18%) required percutaneous drainage, with an accompanying in-hospital mortality of 4.7% (33 of 704 patients), and 177 patients (0.27%) required cardiac surgery, with a higher mortality rate of 11.9% (21 of 177 patients). There were 669 patients (1.02%) who had PE that did not require intervention. Overall, PE was associated with increased risk of MACE including stroke, death, and the composite of death, stroke, and systemic embolism. While the incidence of PE in the large NCDR LAAO registry is less or comparable with the incidence of PE in the PROTECT AF (4.8%), PREVAIL (1.9%), and Amulet IDE trials (1.3%), the morbidity and mortality associated with PE is substantial. Increased operator experience curbs the frequency of PE occurrences. Discharge medication regimens were affected by the occurrence of post-LAAO PE, with over 18% not receiving any form of antithrombotic medication, and a significantly less incidence of anticoagulant prescription, either alone or in combination with an antiplatelet, and more frequent prescription of SAPT or DAPT, when compared with patients without PE. In terms of identifying independent risk factors for PE formation, analysis of the registry data found that older patients, female sex, normal left ventricular function, paroxysmal atrial fibrillation, prior bleeding, lower serum albumin, and preprocedural dual antiplatelet therapy were important clinical variables. Independent risk factors from a procedural standpoint included multiple delivery sheaths used or no delivery sheath, sinus rhythm during the procedure (increased LAA contractile force on the delivery sheath and device), and moderate sedation rather than general anesthesia. The procedural variable most associated with PE was the use of no delivery sheath. The implication is that PE occurred during transseptal puncture before a delivery sheath was placed, suggesting that improvement on the transseptal portion of the procedure may reduce rates of PE. The high mortality rate seen with in-hospital PE requiring intervention indicates that frequency of PE should be included separately in any safety and effectiveness analysis of future devices.
Procedure-Related Stroke
Procedure-related stroke is another rare but dreaded complication of LAAO implantation, given the procedure’s inherent preventive intention. In a retrospective observational registry analysis of 42,114 admissions from 2016 to 2019 from the Nationwide Readmissions Database for LAAO, rates of early stroke (stroke occurring on index hospitalization or 90-day readmission) and early mortality were low at 0.63% (0.13% index admission, 0.50% 90-day readmission) and 0.53%, respectively. These rates are lower than periprocedural stroke rates seen with prospective clinical trials (0.9% to 1.1%). Note that the first-generation Watchman device was the only FDA-approved device from 2016 to 2019. The median time from implant to readmission for stroke was 35 days with an interquartile range of 9 to 57 days. Most readmissions with strokes (67%) occurred less than 45 days after the procedure, prior to cessation of anticoagulation. This could be explained through several mechanisms: 1) microemboli can dislodge during the procedure, 2) thrombi may form during the vulnerable post-procedural period when the LAAO device has yet to endothelialize, and 3) it remains possible that patients are not fully compliant with their post-implantation antithrombotic regimen. Independent clinical variables associated with early stroke after LAAO were peripheral vascular disease and a history of prior stroke. Importantly, early stroke rates after LAAO implantation decreased between the years 2016 (0.64%) and 2019 (0.46%, p < 0.001), with unchanged mortality and MACE events, despite a substantial uptick in LAAO caseloads. In the newer Watchman FLX device, the early stroke rate was noted to be 0.7%, but other retrospective studies comparing the Watchman FLX to the first-generation Watchman show lower early stroke rates in the Watchman FLX. It is expected that low post-procedural stroke rates will continue as newer devices entire the market.
Device Embolization
Device embolization (DE) is an extremely rare complication, with reported rates generally occurring in the 0% to 2% range, with the NCDR LAAO registry citing a rate of 0.07%. (52, 55) , While it was previously thought that a vast majority of DEs occur intraprocedurally, Murtaza et al., pointed out in the Left Atrial Appendage Occlusion Device Embolization (The LAAODE) Study that most cases of DE happened in the postoperative period (61%) compared with intraoperative period (39%). Most cases are recognized in the early postoperative period, with more than 50% of cases discovered in the first 24 hours and 35% cases after one week. DE has been detected up to a year after the procedure. If the DE is detected early, the device is usually still in the LA and the prognosis is much better, as there is a 94% chance of successful percutaneous snaring. If DE is detected late, there is a higher probability of the device being in the LV, LVOT, or aorta, which usually required surgical intervention, and the additional time between implantation and embolization detection allowed for deterioration of the patient’s condition. Major complications were significantly higher with postoperative DE (44.4%) compared with intraoperative DE (22.5%, p=0.03), including higher rates of death in the postoperative group (44.4%; n=28/63) versus intraoperative group (22.5%; n=9/40, p=0.034) and greater need for surgical intervention in the postoperative group (38%; n=23/63) versus the intraoperative group (7.5%; n= 3/40, p=0.016). Symptoms of DE can be nonspecific and insidious, ranging from asymptomatic to palpitations from ectopy and other rhythm disturbances. Consequences of DE can also be far more nefarious, with reported instances of mitral and aortic valve damage, LV outflow tract obstruction, cardiogenic shock, and death. Devices found in the left atrium or aorta are usually retrieved percutaneously and those in the left ventricle are usually retrieved surgically, but percutaneous and surgical means can be used for either retrieval strategy. There are several anatomic, device-related, and procedural risk factors that can create a milieu for DE. Anatomically, if there is a component of device-to-LAA sizing or morphology mismatch, such as an under- or oversized and over-compressed occluder, wide LAA neck, or cactus-shaped LAA, this can lead to DE. High LAA velocities or underfilled LAA can provide unstable local hemodynamic conditions for successful LAAO latching. Procedurally, contributors to mechanical instability can lead to DE, including off-axis or superficial device placement and failure to deploy stabilizing wires, and intrinsic or extrinsic forces such as aggressive tug test, cardioversion, and straining during extubation. Device features that can contribute to the potential for DE include a non-steerable sheath which limits the ability to become coaxial with the LAA once the transseptal puncture site is chosen, and cable malfunction or loose cable-device connections. Prevention of DE involves mitigation of the aforementioned factors, which can be done by employing adequate imaging like CTA and/or TEE for device size planning, cable checks on the device prior to deployment, ensuring adequate filling pressures, and consistently adhering to standardized deployment protocols such as the PASS (position, anchor, size, and seal) criteria. For intervention of DE, it is important to have available extraction snares and other tools immediately present in the interventional lab during the procedure, as well as swift access to cardiothoracic surgery. Performing a 24-hour postoperative transthoracic echocardiogram (TTE) may be helpful in screening for embolization, as these devices are not easily visible on chest x-ray.
Association of Gender With Peri-Procedural Complications
There is a notable association between female sex and in-hospital peri-procedural LAAO complications. Women have twice as much risk of any adverse event or major adverse event compared to men, particularly when it comes to pericardial effusion requiring intervention and major bleeding events. They are also more likely to have an extended hospital stay post-LAAO implantation and death (the absolute risk difference was minimal). Further investigation and greater enrollment of women in trials are needed to help clarify sex-based differences in post-LAAO outcomes.
Post-Procedural Complications
Device-Related Thrombus (DRT)
DRT is one of the most dreaded complications of LAAO device implantation. The goal of LAAO placement is to prevent clot formation and subsequent strokes, so DRT formation on an LAAO device runs counter to this ideal. To prevent DRT formation post-LAAO placement, patients are placed on some combination of antiplatelet or anticoagulation regimen, whether it is aspirin 81 mg daily and warfarin, aspirin and an anticoagulant, or DAPT, for 45 days, after which routine TEE imaging is performed to assess for device endothelialization and formation of thrombus. If there is no thrombus and adequate device seal at 45 days, the antithrombotic regimen can be relaxed to aspirin 81 mg daily and clopidogrel 75 mg daily until 6 months, after which patients can continue on aspirin monotherapy indefinitely. If a thrombus or inadequate seal is seen on the 45-day TEE evaluation, patients continue on warfarin or anticoagulant until an acceptable seal is achieved and the thrombus is resolved. To further investigate the toll of DRT on patients with LAAO, Simard et al., collated a multicenter collaborative registry examining patients with and without DRT and found that patients with DRT after LAAO had a higher association with major adverse cardiovascular events in the form of ischemic events, with no differences in all-cause mortality or systemic embolism. The association of DRT with ischemic events is confirmed in other studies which show 3 to 5-fold increases in strokes in patients with DRT compared with those without. Interestingly, an analysis of the 34 DRT cases in EWOLUTION did not demonstrate a statistical significance between ischemic events in patients with DRT post-LAAO implantation (1.7%) and those without (2.2%, p = 0.80), though the preponderance of evidence strongly supports the relation between DRT and stroke. The incidence of DRT in Simard’s registry was 2.8% (237 cases, 24 centers), with five identified statistically significant risk factors, which included hypercoagulability disorder, pericardial effusion, renal insufficiency, implantation depth >10 mm from the pulmonary vein limbus, and non-paroxysmal AF. Patients with pericardial effusions during the procedure were more likely to not receive post-LAAO antiplatelet or antithrombotic therapies (0.9% versus 9.1%, p = 0.007), though overall post-LAAO medication regimens between the two groups were the same and post-LAAO medication regimen has not been found to be an independent risk factor for DRT formation. At the time of diagnosis of DRT, most patients were on single antiplatelet therapy (SAPT) or DAPT, but after DRT detection, patients were placed back on anticoagulant therapy, which cleared the thrombus on follow-up except for a quarter of the cases. PDL and device migration occurred more often in the DRT cohort. In the 237 DRT cases, which included Watchman, Watchman FLX, Amplatzer ACP, Amplatzer Amulet, and other brand implantations, the thrombus was found on the disk in 131 cases, the screw in 30 cases, next to the device in 20 cases, elsewhere in 11 cases, on the screw and disk in 2 cases, on the disk and next to the device in 2 cases, and unspecified location in 41 cases. The timing of DRT formation was highly variable within a years’ time, with most (64%) being diagnosed less than 180 days post-LAAO implant. The authors stratified the five identified risk factors into major (intraprocedural pericardial effusion and hypercoagulability) and minor (deep implant, renal insufficiency, non-paroxysmal AF) categories, with the presence of 1 major risk factor or 2 minor risk factors conferring a nearly 2-fold increase in the risk for developing DRT post-LAAO. The need to identify patients at higher risk for DRT will continue to be an important issue as implantation rates increase with expanding FDA indications, device models, and increased usage in younger, lower-risk patients. From a proceduralist’s perspective, the only technically modifiable factor to prevent DRT is considering depth of implantation, with the thought that the exposed LAA surface is static enough to foster thrombus formation. Coverage of the pulmonary limbus during implantation was previously suspected and shown to be a factor in reducing DRT rates with use of disk-and-lobe devices, but has yet to be shown in lobe devices such as the Watchman. Two paradoxical observations from the Simard et al., DRT registry were that while the presence of PDL was higher in the DRT cohort, it was not an independent predictor of DRT. Additionally, as previously emphasized, the post-LAAO medication regimen used did not affect formation of DRT. Several attempts are currently being trialed in the materials engineering aspect of LAAO device design to reduce DRT, including reducing exposed metal surfaces to the LAA (as seen in the Watchman FLX) or coating the devices with antithrombotic properties, analogous in principle to the drug-coated layers found on coronary stents. The overall suggestion is that the formation of DRT is influenced by patient, procedural, and materials design, and efforts in improving these areas will lead to safer procedures over time.
Peridevice Leak
Peridevice leak (PDL), otherwise known as incomplete LAAO, is a common post-procedural complication associated with a higher risk of stroke. There is currently no consensus about how to describe PDL - definitions of PDL used in trials vary, and the ideal detection and sizing methods have not been established. To make matters more complex, PDLs are nuanced and protean in nature, with the ability to change in size over time. It has been shown that PDLs ≤ 3 mm get smaller over time, whereas those > 3 mm remain unchanged, though whether this pattern is due to differences in methods of leak detection and reporting is uncertain. It is also important to note the imaging modality used to detect PDLs in trials and in the clinical setting. While TEE was used in the trials to detect PDL, several studies now corroborate that CT is the more sensitive method of PDL detection. CT provides detailed insight into the PDL size, shape, and mechanism compared with TEE, which provides information about the size based on the width of the color Doppler jet. The difference is profound; in fact, 346 patients with Amplatzer implantations underwent both TEE and CT at 8 weeks post-procedure, and TEE detected PDL in 110 patients (32%), while CT picked up nearly double that amount of PDL in 210 patients (61%). Another mystery in defining the PDL is the subject of “fabric leaks” or “micro-PDLs” - the phenomenon where there is no visible connection between the LA and the LAA, yet the LAA is not thrombosed off. The clinical significance of these is unknown, and thus treatment, if any, is also unknown. An additional consideration is there are differences in PDL mechanism and formation between lobe occluders (i.e., Watchman) and disc-and-lobe occluders (i.e., Amplatzer). PDLs found with Watchman are connections between the LA and the distal LAA, whereas PDLs in disc-and-lobe occluders represent portals of flow between either the LA and the distal LAA, or between the LA and the space in between the disc and the lobe. The clinical significance of the different range of PDLs found with the disc-and-lobe devices is currently unknown. The device design differences likely accounted for the higher incidence of PDL in the Watchman 2.5 device versus the Amplatzer in the Amulet IDE trial, though rates of stroke at 18 months were identical in the trial. Finally, while size is the most commonly cited characteristic of a PDL, delineating its mechanism is also crucial for determining treatment strategies, and both parameters should be noted in a description of a PDL. Lobe occluders can manifest edge leaks, uncovered lobe leaks, uncovered proximal LAA lobe leaks, and fabric leaks. Disc-and-lobe occluders can have leaks between disc and lobe, leaks between LA and distal LAA, uncovered proximal LA leaks, and also fabric leaks.
Though once thought to be benign, three large analyses demonstrate PDL and its higher risk of stroke. In the large NCDR LAAO Registry with greater than 50,000 patients, large leaks (> 5 mm) were uncommon (< 1%) and small leaks (< 5 mm) were common (25%) on 45-day TEE. The small PDLs had a higher risk of stroke, systemic embolism, bleeding, and major adverse events. In an analysis of PROTECT AF, PREVAIL, and the CAP registries, persistent small leaks (< 5 mm) had twice as high a risk of stroke and systemic embolism. In a subgroup analysis of the Amulet IDE trial, PDL ≥ 3 mm on 45-day TEE were associated with higher chances of stroke, systemic embolism, and death. PDL has also been linked with a higher risk of bleeding, with small leaks (< 5 mm) showing a higher bleeding risk in the NCDR LAAO Registry and large leaks (> 5 mm) having a higher though statistically insignificant bleeding risk in the Amulet IDE trial. The rationale for the association with bleeding is unknown and warrants further investigation given its potential impact on antithrombotic medication strategies.
Preventing PDL is a priority given its consequences, though many associated risk factors are nonmodifiable. Factors associated with atrial myopathy, including a higher CHA2DS2-VASc score, larger LAA diameter, complex LAA lobe anatomy, and left ventricular cardiomyopathy are linked with PDL, in addition to patients with nonparoxysmal AF. Potentially modifiable factors that increase incidence of PDL include use of lobe devices i.e., Watchman, device undersizing, and inability to gain good co-axial alignment with the LAA. Pre-procedural software programs may be considered by the structuralist to help combine imaging with computational modeling to determine the best sizing and device. The FEops HEARTguide is an interactive tool that combines preprocedural CT with manual overlays of different device sizes, and was recently studied in a randomized trial using the Amplatzer Amulet Device. The 100 patients who had FEops HEARTguide in their planning had more complete closures than the 100 patients who underwent standard CT preprocedural planning. Newer generational devices and steerable sheaths will theoretically mitigate some risk of PDL.
Diagnosing a PDL leads to a decision tree of whether this patient can be managed with watchful waiting, frequent imaging, and long-term anticoagulation (given incomplete closure of the LAA), or whether this patient would benefit from closure. If the patient would benefit from closure, such as a patient unable to tolerate long-term anticoagulation, there is no consensus on when to close (small leaks < 5 mm can demonstrate regression over time), and indications for PDL closure have varied widely among countries and specialties. There is no agreement on which size of PDL is clinically significant (i.e., any leak, only those > 3 mm, only those > 5 mm, etc.). There are not many PDL closures performed overall (< 400 cases in the literature), relative to the number of LAAO procedures performed, and thus there is a paucity of long-term efficacy data.
If the decision is made to intervene, there is a portfolio of interventional approaches to choose from, including vascular plugs, endovascular coils, radiofrequency ablation, or combinations of strategies, and some of these strategies will be familiar to the structuralist who has had to tackle a paravalvular leak for a mechanical or bioprosthetic valve. The Amplatzer Vascular Plug II and Amplatzer Duct Occluder (Abbott Vascular) are choice cardiovascular plugs that can be delivered with a steerable sheath, coronary guide, and an angled Glidewire. The occluders rely on oversizing with a good compression of ≥ 50% for stability, and either more than one occluder may be needed or even a second LAAO device, depending on the size of the leak. Endovascular coils are designed for narrow, shallow leaks that are too small for vascular occluders to fit. Coils are delivered via smaller 4- or 5-Fr catheters or microcatheters, usually via the Azur system (Terumo), with a framing coil base and expansible Azur HydroCoils to fill the LAA. When considering whether to target the PDL neck or fill the LAA, operators tend to occlude the LAA due to coil stabilization from the LAAO device itself. Radiofrequency ablation (RFA) is another avenue for treating PDL. Under moderate or general anesthesia, standard 8-Fr radiofrequency ablation catheters can be traced to the atrial side of the leak through a steerable sheath, with repeated 15-20 second applications of 40-47 Watts of energy. Accidentally wedging the catheter in between the LAAO device and the atrial tissue may cause steam pops or atrial tissue perforation, so monitoring contact force and looking for sudden impedance drops is essential with this method. Edema produced during the procedure may resolve over time and lead to recurrent PDL, so conducting overlapping RFA lesions on the LAA ostium will help increase the efficacy of the procedure. In an observational study of 160 patients comparing successful closure rates of vascular plugs, RFA, and embolization coils on a 45-day TEE in patients requiring leak closure after LAAO, 100% of the vascular plug group had successful closure (defined as no leak or <1mm leak), and 93% in the RFA group and 91% of the embolization coil group had successful closure (p=0.0005). Uncertainties surround what is best practice for post-PDL imaging surveillance and antithrombotic regimen, however, patients typically receive one follow-up study and continue on antiplatelet agents. There appear to be low ischemic rates post-PDL closure, but there are too few patients with PDL closure to draw conclusions at this time. Furthermore, the impact of multiple LAAO devices on DRT has not been investigated yet and would be an interesting future study as caseloads rise.
Left Atrial Pressure and Compliance after Percutaneous Left Atrial Appendage Closure
With the increasing usage of LAAO devices and its projected continued rise, work has been done to reinvestigate the physiologic role of the LAA and its role in LA compliance, and specifically the hemodynamic consequences of LAAO. The LAA is autonomically innervated and is thought to act like a decompression chamber, releasing atrial and brain natriuretic peptides (ANP and BNP) in response to stretch, which inhibit sodium uptake in the kidneys and cause diuresis. Animal studies show that the LAA has increased distensibility compared to the LA and that ligation of the appendage acutely reduces left atrial compliance. In fact, in 22 patients receiving the Amulet, a measure of pre-implantation LA pressure and post-implantation LA pressure (without contrast use so as not to confound pressure measurements) found that as early as 5 minutes post-LAAO deployment, there was a significant rise in left atrial pressure from 12.5 ± 3.9 mm Hg to 19.5 ± 5.1 mm Hg (p = 0.012). The presence of atrial fibrillation did not change the fact that LA pressures rose. Specifically on the left atrial pressure waveform, the increase in the amplitude of the v wave was the greatest contributor to the rise in the left atrial pressure. In another study conducted with simultaneous three-dimensional TEE and direct invasive LA pressure measurements, LA stiffness significantly increased after device implantation. An additional finding was that the magnitude of LA stiffness increase after LAAO was significantly higher in patients with larger LAAs than those with smaller LAAs (P < 0.01). This noted increase in LA pressures post-LAAO has led some authors to study whether LAAO itself could predispose higher-risk patients to more heart failure exacerbations. In a small study of 98 patients who had undergone LAAO, 16% were readmitted with heart failure exacerbation. Risk factors for HF readmissions included a prior history of HF, higher LV mass, higher E/e’, and abnormal LA strain. Patients with heart failure may derive some moderate benefit in exercise capacity after their LAAO implants due to persistent iatrogenic transseptal leaks that provide left-to-right interatrial shunting. This is theorized to be due to the ability of the shunt to serve as a pop off valve during exertion when there is decreased left-sided compliance, which can help lower left atrial pressure. In summary, the existing data suggest that LAAO leads to an increase in LA pressure and a decrease in LA compliance, which could exacerbate heart failure in the correct clinical setting. Additional studies to help identify those at highest risk will aid in selecting the best candidates for this procedure. Importantly, recognizing the potential risks might prompt more aggressive heart failure management and monitoring after device implantation. Using therapeutic atrial shunts to counteract elevations in left atrial pressure after appendage occlusion warrants further study.
Cost Effectiveness of LAAO
By 2025, the LAAO market size is projected to reach $2 billion, which reflects the growing economic burden of atrial fibrillation and the burgeoning medical device market. When patients engage in shared decision-making with their physicians about whether to choose anticoagulation or LAAO device to reduce their stroke risk with atrial fibrillation, multiple factors are at play, including bleeding risk, quality of life, the inconvenience of taking medications, and a consideration of lifetime risks, side effects, and hospital costs. In order to shed light on these important questions, Reddy et al., examined the cost-effectiveness of LAAO in comparison with warfarin and the non–vitamin K oral anticoagulants dabigatran 150 mg, apixaban, and rivaroxaban in the prevention of stroke in nonvalvular atrial fibrillation patients with a prior stroke or transient ischemic attack. Within 10 years, LAAO was found to deliver the best effectiveness and lowest cost compared with warfarin and the NOACs. LAAO had more quality-adjusted life years (QALY) (4.986) versus 4.769, 4.869, 4.888, and 4.810 in the warfarin, dabigatran, apixaban, and rivaroxaban, respectively, and additionally touted lower costs ($42 616 versus $53 770, $58 774, $55 656, and $58 655). It takes time for LAAO to become cost-effective relative to the anticoagulants, with LAAO achieving this relative to dabigatran at year 5 and warfarin and apixaban at year 6. Rivaroxaban was the least effective and most costly of the anticoagulants and thus was excluded from further analysis. At year 10, LAAO superseded warfarin, dabigatran, and apixaban in cost-effectiveness, and this effect persisted into year 20. In the first few years after implantation, LAAO was expensive relative to anticoagulants, but thereafter cost rates slowed and started becoming less costly during years 5 through 7. In another analysis by Freeman et al., LAAO closure was cost effective 90% of the time in the PROTECT AF trial and 9% of the time in the PREVAIL trial when compared directly with warfarin and indirectly with dabigatran, measured at a willingness-to-pay threshold of $50 000 per QALY. The PROTECT AF trial produced more reliable cost-effectiveness results due to its longer-term follow-up and greater enrollment numbers. These cost-effectiveness comparisons for LAAO and anticoagulation took into account rates of hemorrhagic and ischemic stroke, underscoring the importance of maintaining long-term registries to follow up on adverse events, as these continue to impact the final cost-effectiveness ratios. European groups have also examined the cost-effectiveness of LAAO, including Sweden, whose healthcare system is universal for all citizens. Labori et al., conducted a meta-analysis of 7519 individuals with AF who had contraindications to anticoagulation and examined the cost-effectiveness of LAAO versus standard stroke prevention (no antithrombotic treatment) from both a Swedish healthcare and public sector angle. Healthcare costs included those from inpatient and primary care settings, and public sector costs included things such as home care and accommodative housing. The total cost of LAAO implantation, with its subsequent follow-ups and post-procedural care, was 14 984 EUR in the first year. As seen in previous cost-effectiveness analyses, LAAO has lower costs in subsequent years relative to standard of care. The mean cost per patient was 19 032 EUR (LAAO) versus 15 022 EUR (standard of care) from a healthcare perspective (+4010 EUR incremental cost for LAAO), whereas the average cost per patient over a lifetime was projected to be 21 029 EUR (LAAO) and 31 281 EUR (standard of care) from a public sector perspective (-10 252 EUR incremental cost for LAAO). Cost-effectiveness analyses showed that LAAO was more costly yet more effective from a healthcare perspective, and dominant (less costly and more effective than standard of care) from a public sector viewpoint. Additionally, over a lifetime, LAAO had more QALYs per patient than standard of care, primarily due to reduction in risk of first ischemic stroke, and if stroke occurred, they were less disabling in the LAAO group than standard of care. Overall, this study suggests that while LAAO is more costly from a healthcare vantage and less costly from a public sector angle, it is cost-effective from both standpoints and also has a higher QALY rate for patients with AF and contraindications to anticoagulation.
In terms of billing and coding for LAAO in the United States, the Center for Medicare Services mandates that patients receiving LAAO must meet the following criteria: 1) have a high stroke risk (CHADS2 ≥ 2 or CHA2DS2-VASc score ≥ 3), 2) have documentation from a non-procedural physician of a shared discussion of the risks and benefits of LAAO, and 3) have the ability to take short-term anticoagulation but not long-term anticoagulation since LAAO is still considered second line therapy to anticoagulation. The procedure must be performed by an experienced interventionalist, electrophysiologist, or cardiac surgeon who has performed ≥ 25 interventional cardiac procedures involving TSP and continues to maintain skills in performing ≥ 25 interventional cardiac procedures (only 12 of which much be LAAO), over a 2-year period. The device must be implanted at a center that reports its outcomes in a national, audited registry. As the LAAO landscape continues to evolve, more will be known about newer indications and cost-effectiveness of LAAO, which will in turn influence payment requirements and models for the procedure.
Future Directions
From its birth in the surgical realm to its intersection with anticoagulation as a stroke prevention strategy and adoption in the percutaneous field, the concept of LAAO has been better studied and understood over the past two decades, while unearthing further mysteries and challenges that are yet to be grappled with.
Patient selection. At the heart of this discussion are the patients whom LAAO serves. In the modern era of LAAO, the summation of data supports LAAO as noninferior to anticoagulation, and both the latest 2019 AHA/ACC/HRS and 2020 ESC/EACT guidelines support LAAO as a Class IIb, Level of Evidence B in patients with AF with increased risk of stroke, who have contraindications to anticoagulation. While these are the guidelines, there are always embedded caveats, as patient selection for LAAO is becoming increasingly nuanced. The scales must weigh the benefits of LAAO in stroke prevention and bleeding reduction versus the risks of DRT and PDL and the possibility of further ischemic stroke, in contrast with the non-procedural anticoagulation route and its avoidance of invasiveness but its downsides of cost, inconvenience, and rampant incompliance. The physician has risk scores, but ultimately a patient-physician discussion on perceived risk of anticoagulation prompts LAAO consideration. The expected duration of anticoagulation may become more forefront in risk calculations, especially considering younger patients who are initially low bleeding risk but cumulatively gain risk over the years. On the other end of the age spectrum, considering older patients and their expected benefits from LAAO is paramount in clinic discussions, as the beneficial effects of LAAO are known to accumulate over time (non-procedural bleeding events reduced at approximately 6 months in the 4-year follow-up of PRAGUE-17). Treatment futility in LAAO has been studied. In a multicenter study of 807 patients who received LAAO in 8 centers in Europe and Canada from 2009 to 2019, nearly 1 in 6 patients (125 total, 15.5%) died within the first year of their procedure; these deaths were not associated with the success of the procedure or with post-procedural antithrombotic regimens. These patients more closely resembled real-life patient populations, in contrast to more carefully hand-picked patients in trials like PROTECT-AF with multiple exclusion criteria. Identified risk factors independently associated with early death were found to be older age (mean 78 years), lower body mass index, and comorbidities such as diabetes, heart failure, and kidney disease. The authors found that if there were greater than 3 of these risk factors present, this conferred an approximately 50% risk of death within 1 year, so a closer look at the risk/benefit ratio in these patients is advised. LAAO procedures differ from other percutaneous structural heart procedures i.e., TAVR, in that these are high-success, low-risk procedures done in relatively lower risk patients, and this study confirms that much of the mortality after LAAO stems from inherent baseline patient characteristics rather than the procedure itself. Interestingly, unlike guideline directions for TAVR or CRT implants, which state that the procedures are contraindicated if there is less than 1-year life expectancy, there is no such suggestion for the LAAO procedure at this time. Continued work to refine ways of identifying patients who are less likely to benefit from LAAO and pinpointing risk factors for certain complications i.e., DRT and PDL, is ongoing.
Device design. An array of new devices are finding their way into investigational device exemption trials, comparing themselves against the existing industry standards Watchman and Amulet, with the hope of targeting a wider variety of LAA anatomies while carrying fewer complications and fewer PDL and DRT rates. As has happened with other devices such as transcatheter valves and stents, it is expected that better materials and construction will improve delivery (i.e., smaller catheters and more targeted transseptal punctures), reduce thrombogenicity, and enhance tissue endothelialization.
Implantation efficiency. Tandem procedures, whether it is LAAO and TAVR, LAAO and AF ablation, or LAA exclusion and cardiac surgery, are appealing to patients and health systems. Healthcare economics and systems of bundled payments will be persuasive drivers behind the practical realities of combined procedures and clinical studies.
Much as patients enjoy efficient treatment, implanting physicians are seeking avenues to improve procedural efficiency. This pressure is a primary motivator for the use of ICE-guided procedures, controlled by the implanting physician, to eliminate the need for additional imaging staff and scheduling. LAAO delivery systems and transseptal puncture systems are also evolving to include procedural efficiencies such as all-in-one large bore radiofrequency transseptal access sheaths with dilator and combined LAAO device delivery capability. Minimizing procedural time, complexity, and staffing needs will push the field toward additional innovation.
Peri-implant management. With recent 2022 FDA approval for DAPT immediately post-LAAO and existing European approval for this antithrombotic strategy, it is clear that post-procedural medical management of LAAO continues to shift. With the rising focus on catching and preventing DRT and PDL due to the clinical implications of increased stroke risk, the optimal follow-up imaging modality and timing will need to be further delineated. CT has been widely recognized as superior in assessing LAA geometry pre-procedurally and better at characterizing endothelialization, DRT and PDL post-procedurally. However, CT is currently underutilized in the LAAO pathway due to the specialty equipment and infrastructure required for its use as well as lack of understanding of how to tailor post-implant therapy in response to the extra details CT provides over TEE. Studies to delineate the best antithrombotic strategy to use in cases of poor endothelialization or PDL are lacking to date. Until this evidence is available, post-implant practices will remain quite variable.
Optimizing stroke prevention stratagems. The intention of LAAO is to prevent the stroke risk that is innate to atrial fibrillation and the ultimate goal of LAAO should be to offer a very low risk solution that eliminates the need for post-implant drug therapy, preferably immediately after implant. Device iterations to improve adverse events to less than 1/1000, with procedural success >99% for all anatomies, and eliminate the need for post-implant antithrombotics without risk of DRT are underway but have yet to be realized. The next step toward recommending LAAO as first line therapy will depend if the CHAMPION-AF trial finds LAAO to be a noninferior first-choice instead of anticoagulation even in those tolerant of long-term anticoagulation for stroke prevention. If positive, this trial may open doors to a paradigm shift in how stroke prevention is initially approached for a new atrial fibrillation diagnosis. A different vision for stroke optimization, inspired by the superiority of LAAO exclusion plus anticoagulation (commonly known as “belt and suspenders” approach) over anticoagulation alone in preventing stroke as seen in the LAAOS III study, is that future trial concepts may include combining LAAO with anticoagulation to create a superior stroke-prevention regimen. Yet another vision for stroke optimization and bleeding reduction is illustrated by the results of the PACIFIC-AF trial, a multicenter randomized safety trial comparing asundexian (an oral factor XIa inhibitor) with apixaban, which showed lower bleeding events and a high degree of factor XIa inhibition with asundexian compared with apixaban in patients with atrial fibrillation.
Conclusion
Atrial fibrillation reigns as the most common cardiac rhythm disorder, affecting millions of patients worldwide. Thromboembolic cerebral events are the most significant complication of atrial fibrillation, and the left atrial appendage is the most common site of thrombus origination. Depending on the risk of thromboembolic cerebral events in a particular patient, anticoagulation therapy with SAPT, DAPT, NOAC, or warfarin effectively prevents cerebral events in many patients; however, anticoagulation therapy has limitations as well. Even as novel anticoagulation strategies have made a difference in the stroke prevention armamentarium, mechanical occlusion or exclusion of the LAA has shown potential advantages over anticoagulation therapy, especially over the long-term. To this end, multiple surgical and percutaneous techniques to accomplish LAA occlusion or exclusion are currently on the market and several are emerging. In many centers, transcatheter LAA occlusion is rapidly becoming a standard therapy for stroke prevention across a wide spectrum of patients.
Personal perspective
Horst Sievert
Over the past few years, transcatheter LAA occlusion has evolved from a procedure reserved for those with elevated risk of thrombotic events at extreme bleeding risk into an attractive alternative to oral anticoagulation across a large spectrum of patients. Advances in the currently available devices, imaging strategies, LAA access and transseptal puncture techniques has dramatically reduced the risk of complications. This a truly effective and safe procedure with excellent long-term results, which is the ideal for a preventive procedure.
LAAO has a solid place as a stroke prevention tool for patients with atrial fibrillation who need it, and as new data comes through in the next few years, the indications and the patients that could benefit from LAAO is likely to expand. Continued advances in risk stratification, safer antithrombotic regimens, and newer therapies for atrial fibrillation management are currently underway, and so the LAAO landscape continues to evolve at an exciting pace.
Conflict of interest statement
Daniel H Steinberg:
Consulting and/or Speaking honoraria from Boston Scientific, Terumo Interventional Systems, Medtronic, Edwards LifeSciences, Abbott.
Nicholas S Amoroso:
Consulting fees, travel expenses, and/or study honoraria from JenaValve, Edwards LifeSciences, Boston Scientific,
Stefan C. Bertog, Jennifer Franke, Jens Webe:
None
Horst Sievert:
Consulting fees, travel expenses, and/or study honoraria from Abbott, Access Closure, AGA, Angiomed, Ardian, Arstasis, Avinger, Boston, Bridgepoint, CardioKinetix, CardioMEMS, Coherex, Cordis, CSI, Edwards, EndoCross, EndoTex, ev3, FlowCardia, Gore, Guidant, Invatec, Lumen Biomedical, Kensey Nash, Kyoto Medical, Medtronic, Nitinol Devices and Components (NDC), NMT, OAS, Occlutech, Osprey, Ovalis, Pathway, pfm, PendraCare, Percardia, Remon, Rox Medi no cal, Sadra, Sorin, Spectranetics, SquareOne, St. Jude, Terumo, Viacor, Velocimed, Xtent, Cardiokinetix, Access Closure, Coherex,Velocimed, CoAptus, and Lumen Biomedic.
- Lippi G, Sanchis-Gomar F, and Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2021 16(2): p 217-221
- Estes NA, 3rd, Halperin JL, Calkins H, et al., ACC/AHA/Physician Consortium 2008 Clinical Performance Measures for Adults with Nonvalvular Atrial Fibrillation or Atrial Flutter: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Clinical Performance Measures for Atrial Fibrillation) Developed in Collaboration with the Heart Rhythm Society. J Am Coll Cardiol. 2008 51(8): p 865-84
- Camm AJ, Kirchhof P, Lip GY, et al., Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010 12(10): p1360-420
- January CT, Wann LS, Alpert JS, et al., 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014, 64(21): pe1-76
- Lloyd-Jones D, Adams RJ, Brown TM, et al., Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010, 121(7): p948-54
- Hart RG, Pearce LA, Rothbart RM, et al., Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy, Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol. 2000, 35(1): p183-7
- Healey JS, Connolly SJ, Gold MR, et al., Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012, 366(2): p120-9
- Gage BF, Waterman AD, Shannon W, et al., Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001, 285(22): p2864-70
- Go AS, Hylek EM, Chang Y, et al., Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice. JAMA. 2003, 290(20): p2685-92
- Lip GY, Nieuwlaat R, Pisters R, et al., Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010, 137(2): p263-72
- Pisters R, Lane DA, Nieuwlaat R, et al., A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010, 138(5): p1093-100
- Friberg L, Rosenqvist M, and Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012, 125(19): p2298-307
- Lane DA and Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012, 126(7): p860-5
- Camm AJ, Lip GY, De Caterina R, et al., 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation, Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012, 33(21): p2719-47
- Hart RG, Benavente O, McBride R, et al., Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999, 131(7): p492-501
- Investigators AWGotA, Connolly S, Pogue J, et al., Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006, 367(9526): p1903-12
- Investigators A, Connolly SJ, Pogue J, et al., Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009, 360(20): p2066-78
- Wysowski DK, Nourjah P, and Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007, 167(13): p1414-9
- Bungard TJ, Ghali WA, Teo KK, et al., Why do patients with atrial fibrillation not receive warfarin. Arch Intern Med. 2000, 160(1): p41-6
- Brass LM, Krumholz HM, Scinto JM, et al., Warfarin use among patients with atrial fibrillation. Stroke. 1997, 28(12): p2382-9
- White HD, Gruber M, Feyzi J, et al., Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007, 167(3): p239-45
- Gallagher AM, Rietbrock S, Plumb J, et al., Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis. J Thromb Haemost. 2008, 6(9): p1500-6
- Gage BF, van Walraven C, Pearce L, et al., Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004, 110(16): p2287-92
- Connolly SJ, Ezekowitz MD, Yusuf S, et al., Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009, 361(12): p1139-51
- Wallentin L, Yusuf S, Ezekowitz MD, et al., Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010, 376(9745): p975-83
- Patel MR, Mahaffey KW, Garg J, et al., Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011, 365(10): p883-91
- Granger CB, Alexander JH, McMurray JJ, et al., Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011, 365(11): p981-92
- Giugliano RP, Ruff CT, Braunwald E, et al., Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013, 369(22): p2093-104
- Blackshear JL and Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996, 61(2): p755-9
- Madden JL. Resection of the left auricular appendix; a prophylaxis for recurrent arterial emboli. J Am Med Assoc. 1949, 140(9): p769-72
- Holmes DR Jr, Schwartz RS, Latus GG, et al., A History of Left Atrial Appendage Occlusion. Interv Cardiol Clin. 2018, 7(2): p143-150
- Cox JL. The surgical treatment of atrial fibrillation, IV, Surgical technique. J Thorac Cardiovasc Surg. 1991, 101(4): p584-92
- Maan A and Heist EK. Left Atrial Appendage Anatomy: Implications for Endocardial Catheter-based Device Closure. J Innov Card Rhythm Manag. 2020, 11(7): p4179-4186
- Rajiah P, Alkhouli M, Thaden J, et al., Pre- and Postprocedural CT of Transcatheter Left Atrial Appendage Closure Devices. Radiographics. 2021, 41(3): p680-698
- Di Biase L, Santangeli P, Anselmino M, et al., Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012, 60(6): p531-8
- Hara H, Virmani R, Holmes DR, Jr., et al., Is the left atrial appendage more than a simple appendage? Catheter Cardiovasc Interv. 2009, 74(2): p234-42
- Veinot JP, Harrity PJ, Gentile F, et al., Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997, 96(9): p3112-5
- Healey JS, Crystal E, Lamy A, et al., Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005, 150(2): p288-93
- Dawson AG, Asopa S, and Dunning J. Should patients undergoing cardiac surgery with atrial fibrillation have left atrial appendage exclusion. Interact Cardiovasc Thorac Surg. 2010, 10(2): p306-11
- Atti V, Anantha-Narayanan M, Turagam MK, et al., Surgical left atrial appendage occlusion during cardiac surgery: A systematic review and meta-analysis. World J Cardiol. 2018, 10(11): p242-249
- Hindricks G, Potpara T, Dagres N, et al., 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021, 42(5): p373-498
- Whitlock RP, Belley-Cote EP, Paparella D, et al., Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N Engl J Med. 2021, 384(22): p2081-2091
- Ailawadi G, Gerdisch MW, Harvey RL, et al., Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011, 142(5): p1002-9, 1009 e1
- Welniak C. FDA Approves the AtriClip for Stroke Prevention in Atrial Fibrillation Patients. 2010. [cited 2023 July 1, 2023]
- Salzberg SP, Plass A, Emmert MY, et al., Left atrial appendage clip occlusion: early clinical results. J Thorac Cardiovasc Surg. 2010, 139(5): p1269-74
- Gerdisch MW, Garrett HE, Jr., Mumtaz MA, et al., Prophylactic Left Atrial Appendage Exclusion in Cardiac Surgery Patients With Elevated CHA(2)DS(2)-VASc Score: Results of the Randomized ATLAS Trial. Innovations (Phila), 2022, 17(6): p463-470
- Starck CT, Steffel J, Emmert MY, et al., Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interact Cardiovasc Thorac Surg. 2012, 15(3): p416-8
- Left Atrial Appendage Exclusion for Prophylactic Stroke Reduction Trial (LeAAPS). [cited 2023 July 1, 2023]; Available from: https://classic.clinicaltrials.gov/ct2/show/record/NCT05478304
- January CT, Wann LS, Calkins H, et al., 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019, 140(2): pe125-e151
- Alkhouli M, Rihal CS, and Holmes DR, Jr., Transseptal Techniques for Emerging Structural Heart Interventions. JACC Cardiovasc Interv. 2016, 9(24): p2465-2480
- Sick PB, Schuler G, Hauptmann KE, et al., Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007, 49(13): p1490-5
- Eng MH, Wang DD, Greenbaum AB, et al., Prospective, randomized comparison of 3-dimensional computed tomography guidance versus TEE data for left atrial appendage occlusion (PRO3DLAAO). Catheter Cardiovasc Interv. 2018, 92(2): p401-407
- Simard T, El Sabbagh A, Lane C, et al., Anatomic Approach to Transseptal Puncture for Structural Heart Interventions. JACC Cardiovasc Interv. 2021, 14(14): p1509-1522
- Pasupula DK, Siddappa Malleshappa SK, Munir MB, et al., Combined atrial fibrillation ablation and left atrial appendage occlusion procedure in the United States: a propensity score matched analysis from 2016-2019 national readmission database. Europace. 2023, 25(2): p390-399
- Alkhouli M, Ellis CR, Daniels M, et al., Left Atrial Appendage Occlusion: Current Advances and Remaining Challenges. JACC: Advances. 2022, 1(5): p100136
- Omran H, Tzikas A, Sievert H, et al., A History of Percutaneous Left Atrial Appendage Occlusion with the PLAATO Device. Interv Cardiol Clin. 2018, 7(2): p137-142
- Ostermayer SH, Reisman M, Kramer PH, et al., Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J Am Coll Cardiol. 2005, 46(1): p9-14
- Block PC, Burstein S, Casale PN, et al., Percutaneous left atrial appendage occlusion for patients in atrial fibrillation suboptimal for warfarin therapy: 5-year results of the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) Study. JACC Cardiovasc Interv. 2009, 2(7): p594-600
- Holmes DR, Reddy VY, Turi ZG, et al., Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009, 374(9689): p534-42
- Reddy VY, Doshi SK, Sievert H, et al., Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2,3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013, 127(6): p720-9
- Alli O, Doshi S, Kar S, et al., Quality of life assessment in the randomized PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) trial of patients at risk for stroke with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2013, 61(17): p1790-8
- Reddy VY, Sievert H, Halperin J, et al., Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014, 312(19): p1988-98
- Holmes DR Jr, Kar S, Price MJ, et al., Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014, 64(1): p1-12
- Majule DN, Jing C, Rutahoile WM, et al., The Efficacy and Safety of the WATCHMAN Device in LAA Occlusion in Patients with Non-Valvular Atrial Fibrillation Contraindicated to Oral Anticoagulation: A Focused Review. Ann Thorac Cardiovasc Surg. 2018, 24(6): p271-278
- Whitlock RP, Healey JS, and Holmes DR. Left atrial appendage occlusion debate revisited. Circulation. 2015, 131(8): p756-61
- Holmes DR Jr, Reddy VY, Gordon NT, et al., Long-Term Safety and Efficacy in Continued Access Left Atrial Appendage Closure Registries. J Am Coll Cardiol. 2019, 74(23): p2878-2889
- Price MJ, Reddy VY, Valderrabano M, et al., Bleeding Outcomes After Left Atrial Appendage Closure Compared With Long-Term Warfarin: A Pooled, Patient-Level Analysis of the WATCHMAN Randomized Trial Experience. JACC Cardiovasc Interv. 2015, 8(15): p1925-1932
- Reddy VY, Doshi SK, Kar S, et al., 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol. 2017, 70(24): p2964-2975
- Kar S, Doshi SK, Sadhu A, et al., Primary Outcome Evaluation of a Next-Generation Left Atrial Appendage Closure Device: Results From the PINNACLE FLX Trial. Circulation. 2021, 143(18): p1754-1762
- Doshi SK, Kar S, Sadhu A, et al., Two-Year Outcomes With a Next-Generation Left Atrial Appendage Device: Final Results of the PINNACLE FLX Trial. J Am Heart Assoc. 2023, 12(4): pe026295
- Reddy VY, Mobius-Winkler S, Miller MA, et al., Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013, 61(25): p2551-6
- Boersma LV, Ince H, Kische S, et al., Evaluating Real-World Clinical Outcomes in Atrial Fibrillation Patients Receiving the WATCHMAN Left Atrial Appendage Closure Technology: Final 2-Year Outcome Data of the EWOLUTION Trial Focusing on History of Stroke and Hemorrhage. Circ Arrhythm Electrophysiol. 2019, 12(4): pe006841
- Turagam MK, Reddy VY, and Dukkipati SR. EWOLUTION of Watchman Left Atrial Appendage Closure to Patients With Contraindication to Oral Anticoagulation. Circ Arrhythm Electrophysiol. 2019, 12(4): pe007257
- Holmes DR, Reddy VY, Buchbinder M, et al., The Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation (ASAP-TOO) trial. Am Heart J. 2017, 189: p68-74
- Neale T. Watchman FLX Gets DAPT Option Added to Label. 2022. [cited 2023 July 1, 2023]
- CHAMPION-AF Clinical Trial. (CHAMPION-AF). 2023. [cited 2023 July 1, 2023]
- Osmancik P, Herman D, Neuzil P, et al., 4-Year Outcomes After Left Atrial Appendage Closure Versus Nonwarfarin Oral Anticoagulation for Atrial Fibrillation. J Am Coll Cardiol. 2022, 79(1): p1-14
- Meier B, Stegink W, and Tzikas A. History of Percutaneous Left Atrial Appendage Occlusion with AMPLATZER Devices. Interv Cardiol Clin. 2018, 7(2): p151-158
- Meier B, Palacios I, Windecker S, et al., Transcatheter left atrial appendage occlusion with Amplatzer devices to obviate anticoagulation in patients with atrial fibrillation. Catheter Cardiovasc Interv. 2003, 60(3): p417-22
- Asmarats L and Rodes-Cabau J. Percutaneous Left Atrial Appendage Closure: Current Devices and Clinical Outcomes. Circ Cardiovasc Interv. 2017, 10(11)
- Park JW, Bethencourt A, Sievert H, et al., Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv. 2011, 77(5): p700-6
- Kefer J, Vermeersch P, Budts W, et al., Transcatheter left atrial appendage closure for stroke prevention in atrial fibrillation with Amplatzer cardiac plug: the Belgian Registry. Acta Cardiol. 2013, 68(6): p551-8
- Wiebe J, Bertog S, Franke J, et al., Safety of percutaneous left atrial appendage closure with the Amplatzer cardiac plug in patients with atrial fibrillation and contraindications to anticoagulation. Catheter Cardiovasc Interv. 2014, 83(5): p796-802
- Tzikas A, Shakir S, Gafoor S, et al., Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention. 2016, 11(10): p1170-9
- Freixa X, Abualsaud A, Chan J, et al., Left atrial appendage occlusion: initial experience with the Amplatzer Amulet. Int J Cardiol. 2014, 174(3): p492-6
- Abualsaud A, Freixa X, Tzikas A, et al., Side-by-Side Comparison of LAA Occlusion Performance With the Amplatzer Cardiac Plug and Amplatzer Amulet. J Invasive Cardiol. 2016, 28(1): p34-8
- Lakkireddy D, Thaler D, Ellis CR, et al., Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE): A Randomized, Controlled Trial. Circulation. 2021, 144(19): p1543-1552
- Price MJ, Ellis CR, Nielsen-Kudsk JE, et al., Peridevice Leak After Transcatheter Left Atrial Appendage Occlusion: An Analysis of the Amulet IDE Trial. JACC Cardiovasc Interv. 2022, 15(21): p2127-2138
- Galea R, De Marco F, Meneveau N, et al., Amulet or Watchman Device for Percutaneous Left Atrial Appendage Closure: Primary Results of the SWISS-APERO Randomized Clinical Trial. Circulation. 2022, 145(10): p724-738
- Della Rocca DG, Magnocavallo M, Gianni C, et al., Procedural and short-term follow-up outcomes of Amplatzer Amulet occluder versus Watchman FLX device: A meta-analysis. Heart Rhythm. 2022, 19(6): p1017-1018
- Prevention of Stroke by Left Atrial Appendage Closure in Atrial Fibrillation Patients After Intracerebral Hemorrhage. [cited. 2023 July 1. 2023]
- Amplatzer Amulet LAAO vs. NOAC (CATALYST). [cited. 2023 September 6, 2023]
- B W. WaveCrest. in Transcatheter Cardiovascular Therapeutics. 2014, Washington, DC
- Reddy VY ea. Clinical experience with the Wavecrest LA appendage occlusion device for stroke prevention in AF: acute results of the WAVECREST I trial. in The Heart Rhythm Society’s 35th Annual Scientific Sessions. 2014, San Francisco, CA
- WAVECREST Post Market Clinical Follow-Up (PMCF) Study. 2022 [cited 2023 July 1, 2023]; Available from: https://classic.clinicaltrials.gov/ct2/show/NCT03204695
- WAveCrest Vs. Watchman TranssEptal LAA Closure to REduce AF-Mediated STroke 2 (WAVECREST2). 2023 [cited 2023 July 1, 2023]; Available from: https://clinicaltrials.gov/study/NCT03302494?tab=results
- Pagnotta PA, Chiarito M, Pllaha E, et al., Left atrial appendage closure with the Ultraseal device: Initial experience and mid-term follow-up. J Interv Cardiol. 2018, 31(6): p932-938
- Ahlgrimm B, Pottgiesser T, Stachon P, et al., The Cardia Ultraseal Left Atrial Appendage Occluder: A Case Series With Significant Device-Related Complications. JACC Cardiovasc Interv. 2019, 12(19): p1987-1989
- Pivato CA, Liccardo G, Sanz-Sanchez J, et al., Left atrial appendage closure with the II generation Ultraseal device: An international registry, The LIGATE study. Catheter Cardiovasc Interv. 2022, 100(4): p620-627
- Chow DHF, Wong YH, Park JW, et al., An overview of current and emerging devices for percutaneous left atrial appendage closure. Trends Cardiovasc Med. 2019, 29(4): p228-236
- H S. Clinical experience with the L'Ambre left atrial appendage occlusion system. in Joint Interventional Meeting. 2017, Milan, Italy
- Huang H, Liu Y, Xu Y, et al., Percutaneous Left Atrial Appendage Closure With the LAmbre Device for Stroke Prevention in Atrial Fibrillation: A Prospective, Multicenter Clinical Study. JACC Cardiovasc Interv. 2017, 10(21): p2188-2194
- Wong GX and Singh GD. Transcatheter Left Atrial Appendage Closure. Methodist Debakey Cardiovasc J. 2023, 19(3): p67-77
- Bartus K, Han FT, Bednarek J, et al., Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. 2013, 62(2): p108-118
- Price MJ, Gibson DN, Yakubov SJ, et al., Early safety and efficacy of percutaneous left atrial appendage suture ligation: results from the US transcatheter LAA ligation consortium. J Am Coll Cardiol. 2014, 64(6): p565-72
- Bartus K, Gafoor S, Tschopp D, et al., Left atrial appendage ligation with the next generation LARIAT(+) suture delivery device: Early clinical experience. Int J Cardiol. 2016, 215: p244-7
- Wilber DJ LD. Outcomes Of Adjunctive Left Atrial Appendage Ligation Utilizing The LARIAT Compared To Pulmonary Vein Antral Isolation Alone: The aMAZE Trial. in American Heart Association (AHA). 2021, Virtual
- LAMINAR. Available from. https://laminarlaa.com/
- Next Generation LAA Elimination System. Available from: https://www.appendmedical.com/
- Price MJ, Valderrabano M, Zimmerman S, et al., Periprocedural Pericardial Effusion Complicating Transcatheter Left Atrial Appendage Occlusion: A Report From the NCDR LAAO Registry. Circ Cardiovasc Interv. 2022, 15(5): pe011718
- Kogan EV, Sciria CT, Liu CF, et al., Early Stroke and Mortality After Percutaneous Left Atrial Appendage Occlusion in Patients With Atrial Fibrillation. Stroke. 2023, 54(4): p947-954
- Freeman JV, Varosy P, Price MJ, et al., The NCDR Left Atrial Appendage Occlusion Registry. J Am Coll Cardiol. 2020, 75(13): p1503-1518
- Alkhouli M, Sievert H, and Rihal CS. Device Embolization in Structural Heart Interventions: Incidence, Outcomes, and Retrieval Techniques. JACC Cardiovasc Interv. 2019, 12(2): p113-126
- Murtaza G, M KT, Dar T, et al., Left Atrial Appendage Occlusion Device Embolization (The LAAODE Study): Understanding the Timing and Clinical Consequences from a Worldwide Experience. J Atr Fibrillation. 2021, 13(5): p2516
- Darden D, Duong T, Du C, et al., Sex Differences in Procedural Outcomes Among Patients Undergoing Left Atrial Appendage Occlusion: Insights From the NCDR LAAO Registry. JAMA Cardiol. 2021, 6(11): p1275-1284
- Simard T, Jung RG, Lehenbauer K, et al., Predictors of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion. J Am Coll Cardiol. 2021, 78(4): p297-313
- Freixa X, Cepas-Guillen P, Flores-Umanzor E, et al., Pulmonary ridge coverage and device-related thrombosis after left atrial appendage occlusion. EuroIntervention. 2021, 16(15): pe1288-e1294
- Alkhouli M, De Backer O, Ellis CR, et al., Peridevice Leak After Left Atrial Appendage Occlusion: Incidence, Mechanisms, Clinical Impact, and Management. JACC Cardiovasc Interv. 2023, 16(6): p627-642
- Afzal MR, Gabriels JK, Jackson GG, et al., Temporal Changes and Clinical Implications of Delayed Peridevice Leak Following Left Atrial Appendage Closure. JACC Clin Electrophysiol. 2022, 8(1): p15-25
- Korsholm K, Jensen JM, Norgaard BL, et al., Peridevice Leak Following Amplatzer Left Atrial Appendage Occlusion: Cardiac Computed Tomography Classification and Clinical Outcomes. JACC Cardiovasc Interv. 2021, 14(1): p83-93
- Charate R, Ahmed A, Della Rocca DG, et al., Evaluation of Multimodality LAA Leak Closure Methods Following Incomplete Occlusion: The LAA Leak Study. JACC Cardiovasc Interv. 2022, 15(21): p2158-2170
- Sharma E, Apostolidou E, Sheikh W, et al., Hemodynamic effects of left atrial appendage occlusion. J Interv Card Electrophysiol. 2022, 64(2): p349-357
- Hoit BD and Walsh RA. Regional atrial distensibility. Am J Physiol. 1992, 262(5 Pt 2): pH1356-60
- Hoit BD, Shao Y, Tsai LM, et al., Altered left atrial compliance after atrial appendectomy. Influence on left atrial and ventricular filling. Circ Res, 1993, 72(1): p167-75
- Patti G, Rapacciuolo A, Segreti A, et al., Percutaneous Left Atrial Appendage Closure: Acute Effects on Left Atrial Pressure in Humans. JACC Cardiovasc Interv. 2019, 12(11): p1089-1091
- Bregasi A, Freeman JV, Curtis JP, et al., Abnormal left atrial body stiffness is predicted by appendage size: impact of appendage occlusion on left atrial mechanics assessed by pressure-volume analysis. Am J Physiol Heart Circ Physiol. 2022, 323(3): pH559-H568
- Kim DY, Kim MJ, Seo J, et al., Predictors of Subsequent Heart Failure After Left Atrial Appendage Closure. Circ J. 2022, 86(7): p1129-1136
- Mitrega K, Streb W, Szymala M, et al., The influence of iatrogenic interatrial septum leaks after left atrial appendage closure on cardiac function test results. J Interv Cardiol. 2018, 31(5): p679-684
- Mohan RC and Litwin SE. The Intriguing Links Among Patent Foramen Ovale, Patent Foramen Ovale Closure, and the Risk for Heart Failure. J Am Soc Echocardiogr. 2023
- Reddy VY, Akehurst RL, Amorosi SL, et al., Cost-Effectiveness of Left Atrial Appendage Closure With the WATCHMAN Device Compared With Warfarin or Non-Vitamin K Antagonist Oral Anticoagulants for Secondary Prevention in Nonvalvular Atrial Fibrillation. Stroke. 2018, 49(6): p1464-1470
- Freeman JV, Hutton DW, Barnes GD, et al., Cost-Effectiveness of Percutaneous Closure of the Left Atrial Appendage in Atrial Fibrillation Based on Results From PROTECT AF Versus PREVAIL. Circ Arrhythm Electrophysiol. 2016, 9(6)
- Labori F, Persson J, Bonander C, et al., Cost-effectiveness analysis of left atrial appendage occlusion in patients with atrial fibrillation and contraindication to oral anticoagulation. Eur Heart J. 2022, 43(13): p1348-1356
- Percutaneous Left Atrial Appendage (LAA) Closure Therapy. [cited. 2023 July 1, 2023].
- Mesnier J, Cruz-Gonzalez I, Arzamendi D, et al., Incidence and Predictors of Early Death in Patients Undergoing Percutaneous Left Atrial Appendage Closure. JACC Clin Electrophysiol. 2022, 8(9): p1093-1102
- Piccini JP, Caso V, Connolly SJ, et al., Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): a multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet. 2022, 399(10333): p1383-1390.