Interventions for non-ST-segment elevation acute coronary syndromes
Summary
Non-ST-segment elevation acute coronary syndrome (NSTE-ACS) is a leading cause of morbidity and mortality in the western countries. The European Society of Cardiology has 2015 released new guidelines on the treatment of NSTE-ACS. These guidelines summarise the current evidence and its translation into clinical practice. Special attention has been given to diagnostic measures, risk stratification, and different therapeutic options. For the diagnostic work-up in the acute phase, the electrocardiogram (ECG) and the assessment of cardiac biomarkers play the central role. Most important changes relate to the introduction of a rapid new rule-out protocol by use of high- or ultrasensitive troponin assays and the choice of stent type. Furthermore, the diagnostic and therapeutic algorithm has been elaborated by recommendation of a general use of risk and bleeding scores and a diversified therapeutic regimen according to risk stratification. For all patients, administration of an anti-aggregatory therapy, including acetylsalicylic acid and prasugrel or ticagrelor, is indicated in the acute phase, while clopidogrel is only advised in the case of contraindications to either ticagrelor or prasugrel. In the chronic phase, optimal treatment of cardiovascular risk factors is of prime importance. However, in the last years some new data have been published including important evidence regarding the treatment of patients requiring oral anticoagulation, which have not been not considered in the 2015 guidelines.
Introduction
The clinical presentations of CAD include silent ischaemia, stable angina pectoris, unstable angina, myocardial infarction (MI), heart failure, and sudden death. Patients with chest pain present a very substantial proportion of all acute medical hospitalisations in Europe. Distinguishing patients with acute coronary syndromes (ACS) within the very large proportion with suspected cardiac pain presents a diagnostic challenge, especially in individuals without clear symptoms or electrocardiographic features. Despite modern treatment, the rates of death, MI, and readmission of patients with ACS remain high.
It is well established that ACS in their different clinical presentations share a common pathophysiological substrate. Pathological, imaging, and biological observations have demonstrated that atherosclerotic plaque rupture or erosion, with differing degrees of superimposed thrombosis and distal embolization, result in myocardial hypoperfusion, and forms the basic pathophysiological mechanism in most cases of ACS.
As this may be a life-threatening manifestation of atherothrombotic disease, criteria for risk stratification have been developed to allow the clinician to make timely decisions on pharmacological management as well as coronary revascularisation strategies, tailored to the individual patient. The leading symptom that initiates the diagnostic and therapeutic cascade is chest pain/discomfort, but the classification of patients is based on the electrocardiogram (ECG). Two categories of patients may be encountered:
1. Patients with acute chest pain and persistent (>20 min) ST-segment elevation. This is termed ST-elevation ACS (STE-ACS) and generally reflects an acute total coronary occlusion. Most of these patients will ultimately develop an ST-elevation MI (STEMI). The therapeutic objective is to achieve rapid, complete, and sustained reperfusion by primary angioplasty or fibrinolytic therapy (Interventions for ST-segment elevation acute myocardial infarction).
2. Patients with acute chest pain but without persistent ST-segment elevation. These patients have persistent or transient ST-segment depression or T-wave inversion, flat T waves, pseudo-normalisation of T waves, or no ECG changes at presentation. The initial strategy in these patients is to alleviate ischaemia and symptoms, to monitor the patient with serial ECGs, and to repeat measurements of markers of myocardial necrosis. At presentation, the working diagnosis of non-ST-elevation ACS (NSTE-ACS) can be further qualified, based on the measurement of troponins, as non-ST-elevation MI (NSTEMI) or unstable angina (Figure 1). In a certain number of patients, coronary heart disease will subsequently be excluded as the cause of symptoms.
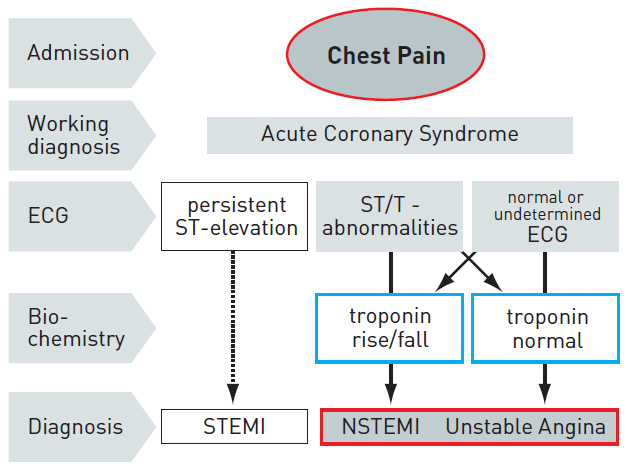
Figure 1
ECG: electrocardiogram; NSTEMI : non-ST-elevation myocardial infarction; STEMI : ST-elevation myocardial infarction.
EPIDEMIOLOGY AND NATURAL HISTORY
Registry data consistently show that NSTE-ACS is more frequent than ST-elevation ACS. The annual incidence is around 3 per 1,000 inhabitants, but varies between countries. Hospital mortality is higher in patients with STEMI than among those with NSTE-ACS (7% vs. 3 % to 5% respectively), but at 6 months the mortality rates are very similar in both conditions (12% and 13% respectively). In fact long-term follow-up showed that death rates were higher among patients with NSTE-ACS than with STE-ACS, with a twofold difference at 4 years . This difference in mid- and long-term evolution may be due to different patient profiles, since NSTE-ACS patients tend to be older, with more comorbidities, especially diabetes and renal failure.
Further data regarding the epidemiology and natural history of NSTE-ACS are also covered in the ESC Textbook of Cardiovascular Medicine.
PATHOPHYSIOLOGY
ACS represents a potentially life-threatening manifestation of atherosclerosis. It is usually precipitated by acute thrombosis induced by a ruptured or eroded atherosclerotic coronary plaque, with or without concomitant vasoconstriction, causing a sudden and critical reduction in myocardial blood flow. In the complex process of plaque disruption, inflammation plays a key pathophysiological role. In rare cases, ACS may have a non-atherosclerotic aetiology such as arteritis, trauma, dissection, thromboembolism, congenital anomalies, cocaine abuse, or complications of cardiac catheterisation. The key pathophysiological concepts of vulnerable plaque, coronary thrombosis, vulnerable patient, endothelial dysfunction, accelerated atherothrombosis, secondary mechanisms of NSTE-ACS and myocardial injury have to be understood in order that correct use of available therapeutic strategies is made. The lesions presenting as ACS are often angiographically mild, characterised by a thin-cap fibroatheroma, by a large plaque burden, or by a small luminal area, or some combination of these characteristics.
Diagnosis
The leading symptom of ACS is typically chest pain. The working diagnosis of NSTE-ACS is a rule-out diagnosis based on the ECG, i.e., lack of persistent ST elevation. Biomarkers (troponins) further distinguish NSTEMI and unstable angina. Imaging modalities are used to rule-out or rule-in differential diagnoses. Diagnosis finding and risk stratification are closely linked.
CLINICAL PRESENTATION
The clinical presentation of NSTE-ACS encompasses a wide variety of symptoms. Traditionally, several clinical presentations have been distinguished:
- Prolonged (>20 min) anginal pain at rest;
- New onset (de novo) angina (Class II or III of the Classification of the Canadian Cardiovascular Society);
- Recent destabilisation of previously stable angina with at least Canadian Cardiovascular Society Class III angina characteristics (crescendo angina); or
- Post MI angina.
The typical clinical presentation of NSTE-ACS is retrosternal pressure or heaviness (“angina”) radiating to the left arm, neck or jaw, which may be intermittent (usually lasting for several minutes) or persistent. These complaints may be accompanied by other symptoms such as diaphoresis, nausea, abdominal pain, dyspnoea, and syncope. However, atypical presentations are not uncommon. These include epigastric pain, indigestion, stabbing chest pain, chest pain with some pleuritic features, or increasing dyspnoea. Atypical complaints are more often observed in older (>75 years) patients, in women, and in patients with diabetes, chronic renal failure, or dementia. Absence of chest pain leads to under-recognition and under-treatment of the disease. The diagnostic and therapeutic challenges arise especially when the ECG is normal or nearly normal, or conversely when the ECG is abnormal at baseline due to underlying conditions such as intraventricular conduction defects or left ventricular (LV) hypertrophy.
Certain features, in terms of the symptoms, may support the diagnosis of CAD and guide patient management. The exacerbation of symptoms by physical exertion, or their relief at rest or after the administration of nitrates, supports a diagnosis of ischaemia. It is important to identify clinical circumstances that may exacerbate or precipitate NSTE-ACS, such as anaemia, infection, inflammation, fever, and metabolic or endocrine (in particular thyroid) disorders.
When faced with a symptomatic patient, the presence of several clinical findings increases the probability of CAD and therefore NSTE-ACS. These include older age, male sex, a positive family history, and known atherosclerosis in non-coronary territories, such as peripheral or carotid artery disease. The presence of risk factors, in particular diabetes mellitus and renal insufficiency as well as prior manifestation of CAD (i.e., previous MI, percutaneous intervention [PCI] or coronary bypass graft surgery [CABG]), also raises the likelihood of NSTE-ACS.
DIAGNOSTIC TOOLS
Electrocardiogram
The resting 12-lead ECG is the first-line diagnostic tool in the assessment of patients with suspected NSTE-ACS. It should be obtained within 10 minutes after first medical contact (either arrival of the patient in the emergency room or first contact with emergency medical services in the pre-hospital setting) and immediately interpreted by a qualified physician. The characteristic ECG abnormalities of NSTE-ACS are ST-segment depression or transient elevation and/or T-wave changes . The finding of persistent (>20 min) ST-elevation suggests STEMI, which mandates an alternative triage and treatment approach (Interventions for ST-segment elevation acute myocardial infarction).
Biomarkers
Cardiac troponins play a central role in establishing a diagnosis and stratifying risk, and make it possible to distinguish between NSTEMI and unstable angina. Troponins are more specific and sensitive than the traditional cardiac enzymes such as creatine kinase (CK), its isoenzyme MB (CK-MB), and myoglobin. Elevation of cardiac troponins reflects myocardial cellular damage, which in NSTE-ACS may result from distal embolisation of platelet-rich thrombi from the site of a ruptured or eroded plaque. In the setting of myocardial ischaemia (chest pain, ECG changes, new wall-motion abnormalities) troponin elevation indicates MI
In patients with MI, an initial rise in troponins occurs within 2 to 4 hours after symptom onset. Troponins may remain elevated for up to 2 weeks due to proteolysis of the contractile apparatus. In NSTE-ACS, minor troponin elevations usually resolve within 48-72 hours.
In the clinical setting, a test with a high ability to rule out (negative predictive value) and correctly diagnose ACS (positive predictive value) is of paramount interest. The diagnostic cut-off for MI is defined as a cardiac troponin measurement exceeding the 99th percentile of a normal reference population (upper reference limit) using an assay with an imprecision (coefficient of variation) of ≤10% at the upper reference limit. Recently, high-sensitivity and ultra-sensitive assays have been introduced that have a 10- to 100-fold lower limit of detection and fulfil the requirements of analytical precision. Therefore, high-sensitivity assays are recommended over less sensitive tests .
Owing to the improved analytical sensitivity, low troponin levels can now also be detected in many patients with chronic coronary syndrome and stable angina and in healthy individuals . The underlying mechanisms of this troponin release are not yet sufficiently elucidated, but any measurable troponin is associated with an unfavourable prognosis. In order to maintain specificity for MI, there is now an emerging need to distinguish chronic from acute troponin elevation. Therefore, the magnitude of change depending on the initial value gains importance to differentiate acute from chronic myocardial damage.
In contrast to other biomarkers, only CK-MB and copeptin seem to have clinical relevance for the diagnosis of NSTE-ACS. CK-MB shows a more rapid decline after MI than cardiac troponin and may provide additional information by detection of early reinfarction. Parallel measurement of copeptin may quantify the endogenous stress level in multiple medical conditions including MI and therefore adds significant value to conventional (particularly less sensitive) cardiac troponin assays to rule out ACS , , .
Due to the higher sensitivity and diagnostic accuracy for the detection of acute MI, the time interval to the second cardiac troponin assessment can be significantly reduced with the use of high-sensitivity assays. As an alternative to the 0 h/3 h algorithm, a 0 h/1 h protocol is recommended when high-sensitivity cardiac troponin assays with a validated algorithm are available. The negative predictive value for MI in patients assigned ‘rule-out’ exceeded 98% in several large validation cohorts. The positive predictive value for MI in those patients meeting the ‘rule-in’ criteria was 75–80% , , , . For rapid rule-out, two alternative approaches to the 0 h/1 h or 0 h/3 h algorithms have been adequately validated and may be considered. First, a 2-h rule-out protocol combining the Thrombolysis in Myocardial Infarction (TIMI) risk score with ECG and high-sensitivity cardiac troponin at presentation allowed a safe rule-out in up to 40% of patients. Second, a dual-marker strategy combining normal levels of cardiac troponin together with low levels of copeptin (10 pmol/l) at presentation had very high negative predictive value for MI , , .
Other life-threatening conditions presenting with chest pain, such as dissecting aortic aneurysm or pulmonary embolism, may also result in elevated troponins and should always be considered in a differential diagnosis. Elevation of cardiac troponins also occurs in the setting of non-coronary-related myocardial injury (Table 1). This reflects the sensitivity of the marker for myocardial cell injury and should not be labelled as a false positive. Truly false-positive results have been documented in the setting of skeletal myopathies or chronic renal failure. Elevated troponin levels are frequently found when the serum creatinine level is >2.5 mg/dL (221 μmol/L) in the absence of proven ACS, and this elevation is associated with an adverse prognosis . However, high-sensitivity cardiac troponin assays also maintain high diagnostic accuracy in patients with renal dysfunction. To ensure the best possible clinical application, it is important to use assay-specific optimal cut-off levels that are higher in patients with renal dysfunction .
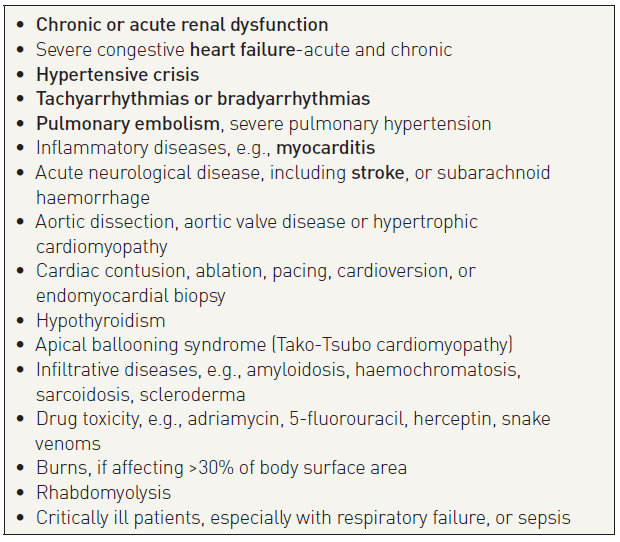
Table 1
Invasive imaging (coronary angiography)
Coronary angiography provides unique information on the presence and severity of CAD and therefore remains the gold standard. It is recommended to perform angiograms before and after intracoronary administration of vasodilators (nitrates) in order to attenuate vasoconstriction and offset the dynamic component that is frequently present in ACS. In haemodynamically compromised patients (e.g., with pulmonary oedema, hypotension, severe life-threatening arrhythmias) it may be advisable to perform the examination after placement of an intra-aortic balloon pump, to limit the number of coronary injections, and to abstain from LV angiography. Angiography should be performed urgently (< 2 hours) for diagnostic purposes in patients at high risk and in whom the differential diagnosis is unclear. The identification of acute thrombotic occlusions (e.g., circumflex artery) is particularly important in patients with ongoing symptoms or relevant troponin elevation but in the absence of diagnostic ECG changes.
Data from the Thrombolysis In Myocardial Infarction (TIMI)-3B and Fragmin during Instability in Coronary Artery Disease-2 (FRISC-2) studies show that 30%-38% of patients with unstable coronary syndromes have single-vessel disease and 44%-59% have multivessel disease (>50% diameter stenosis). The incidence of left main narrowing varies from 4% to 8%. Patients with multivessel disease as well as those with left main stenosis are at the highest risk of serious cardiac events. Coronary angiography in conjunction with ECG findings and regional wall motion abnormalities frequently allows identification of the culprit lesion. Typical angiographic features are eccentricity, irregular borders, ulceration, haziness, and filling defects suggestive of the presence of intracoronary thrombus. In lesions whose severity is difficult to assess, intravascular ultrasound or fractional flow reserve (FFR) measurements carried out more than 5 days after the index event are useful in order to decide on the treatment strategy.
The choice of vascular access site depends on operator expertise and local preference, but due to the large impact of bleeding complications on clinical outcome in patients with elevated bleeding risk, the decision is important. Accordingly, ESC guidelines strongly recommend the radial access for the past several years. This is based on the large MATRIX radial trial: among 8404 patients with NSTEMI or STEMI there was a significant reduction in the incidence of the combined endpoint that included cardiovascular death, stroke, and myocardial infarction using the radial approach as compared with the femoral approach . This was mainly due to a significant reduction in the number of major bleeding events and in overall mortality (1.6% vs. 2.2%, p=0.045). These results were further confirmed by a large meta-analysis . Therefore, the radial access site is preferred in patients at high risk of bleeding provided the operator has sufficient experience with this technique. The radial approach has a lower risk of large hematomas at the price of higher radiation dose for patients and staff . The femoral approach may be preferred in hemodynamically compromised patients to facilitate of the use of extracorporeal membrane oxygenation (ECMO) support.
DIFFERENTIAL DIAGNOSES
Several cardiac and non-cardiac conditions may mimic NSTE-ACS (Table 2). Underlying chronic conditions such as hypertrophic cardiomyopathy and valvular heart disease (i.e., aortic stenosis, aortic regurgitation) may be associated with typical symptoms of NSTE-ACS, elevated cardiac biomarkers, and ECG changes. Sometimes symptoms associated with paroxysmal atrial fibrillation may mimic ACS. Since some of these patients also have CAD, the diagnostic process can be difficult. In addition, myocarditis, pericarditis, or myopericarditis of different aetiologies may be associated with chest pain that resembles the typical angina of NSTE-ACS, and can be associated with a rise in cardiac biomarker levels, ECG changes, and wall motion abnormalities.
In assessment of patients with potential ACS, non-cardiac life-threatening conditions must be ruled out. Among these, pulmonary embolism may be associated with dyspnoea, chest pain, and ECG changes, as well as elevated levels of cardiac biomarkers similar to those of NSTE-ACS. Aortic dissection is the other condition to be considered as an important differential diagnosis. NSTE-ACS may be a complication of aortic dissection when the dissection involves the coronary arteries.
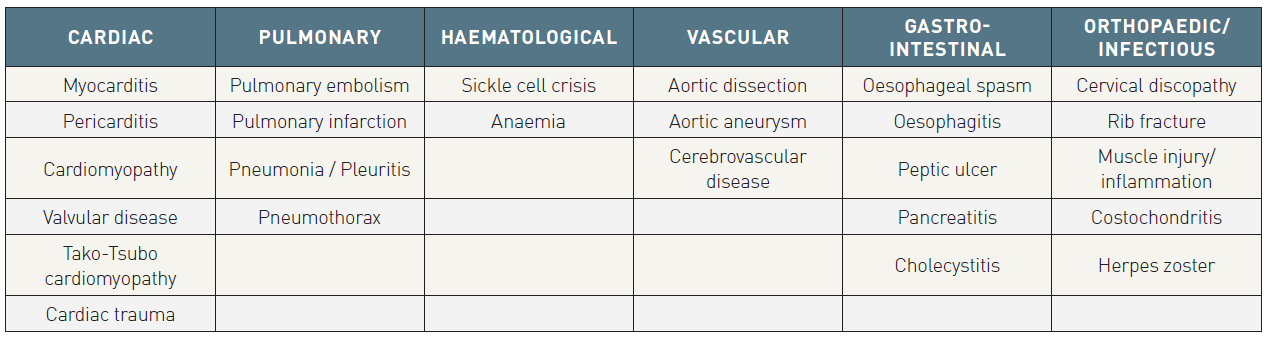
Table 2
Assessment of prognosis
NSTE-ACS is an unstable coronary condition prone to ischaemic recurrences and other complications that may lead to death or MI in the short and long term. The management of this condition, which includes anti-ischaemic and antithrombotic pharmacological treatments as well as various strategies for coronary revascularisation, is directed to prevent or reduce such complications and to improve outcomes. The timing and intensity of these interventions should be tailored to the individual patient’s risk. As many treatment options increase the risk of haemorrhagic complications, this needs to be carefully balanced on an individual basis. Since the spectrum of risk associated with NSTE-ACS is wide and particularly high in the early hours, risk must be carefully assessed immediately after first medical contact. Risk assessment is a continuous process that remains ongoing until hospital discharge and that may result in modification of the treatment strategy at any time. Dedicated chest pain units or coronary care units may improve care of ACS patients.
ELECTROCARDIOGRAM INDICATORS
The initial ECG presentation is predictive of early risk. Patients with a normal ECG on admission have a better prognosis than those with negative T waves. Patients with ST-segment depression have an even worse prognosis, which is dependent on the severity and extent of ECG changes. , The number of leads showing ST depression and the magnitude of ST depression are indicative of the extent and severity of ischaemia and correlate with prognosis. ST-segment depression >0.05 mV in two or more contiguous leads, in the appropriate clinical context, is suggestive of NSTE-ACS and linked to prognosis. More relevant is ST depression of >0.1 mV, which is associated with an 11% rate of death and MI at 1 year. ST depression of >0.2 mV carries about a six-fold increased mortality risk. ST depression combined with transient ST elevation identifies an even higher risk subgroup.
Patients with ST depression have a higher risk for subsequent cardiac events compared to those with isolated T-wave inversion (>0.1 mV) in leads with predominant R-waves, who in turn have a higher risk than those with a normal ECG on admission. Some studies have cast doubt on the prognostic value of isolated T-wave inversion. However, deep symmetrical inversion of the T-waves in the anterior chest leads is often related to a significant stenosis of the proximal left anterior descending coronary artery or main stem.
Other features, such as elevation (>0.1 mV) in lead aVR have been associated with a high probability of left-main or triple-vessel CAD and worse clinical prognosis.
BIOMARKERS
Biomarkers reflect different pathophysiological aspects of NSTE-ACS. Troponin T or I are the preferred biomarkers to predict short-term (30 days) outcome with respect to MI and death. The prognostic value of troponin measurements has also been confirmed over the long term (1 year and beyond). NSTEMI patients with elevated troponin levels but no rise in CK-MB (who comprise approximately 28% of the NSTEMI population), although undertreated, have a higher-risk profile and lower in-hospital mortality than patients with both markers elevated. The increased risk associated with elevated troponin levels is independent of and additive to other risk factors, such as ECG changes at rest or on continuous monitoring, or markers of inflammatory activity. Furthermore, the identification of patients with elevated troponin levels is also useful for selecting appropriate treatment in patients with NSTE-ACS. However, troponins should not be used as sole criterion for risk adjudication, because in-hospital mortality may be as high as 12.7% in certain high-risk troponin-negative subgroups.
Due to low sensitivity for MI, a single negative test on first contact with the patient is not sufficient for ruling out NSTE-ACS, as in many patients an increase in troponins can be detected only in the subsequent hours. Therefore, repeated measurements after 6-9 hours have been advocated. The recently introduced high sensitivity troponin assays better identify patients at risk and provide reliable and rapid prognosis prediction allowing a fast track rule-out protocol (3 hours).
While cardiac troponins are the key biomarkers for initial risk stratification, multiple other biomarkers have been evaluated for incremental prognostic information. Of these, high-sensitivity C-reactive protein (hsCRP) and brain natriuretic peptide (BNP) have both been extensively validated and are routinely available. Elevated hsCRP levels are associated with increased mortality at the time of the index event and continuously increase over the next years. This was also observed in large cohorts of patients undergoing elective PCI. However, hsCRP and BNP have no impact on acute decision making in ACS.
Impaired renal function is a strong independent predictor of long-term mortality in ACS patients. Serum creatinine concentration is a less reliable indicator of renal function than creatinine clearance (CrCl) or estimated glomerular filtration rate (eGFR) because it is affected by a multitude of factors including age, weight, muscle mass, race, and various medications. Several formulae have been devised to improve the accuracy of serum creatinine level as a surrogate for eGFR, including the Cockcroft-Gault and the abbreviated Modification of Diet in Renal Disease (MDRD) equations. Long-term mortality increases exponentially with decreasing eGFR/CrCl (Lesion and patient subsets: chronic kidney disease (old), Contrast agents and renal protection (old)).
RISK SCORES
Quantitative assessment of risk is useful for clinical decision-making. Several scores have been developed from different populations to estimate ischaemic and bleeding risks, with different outcomes and time frames. In clinical practice simple risk scores may be more convenient and preferred.
Risk scores of outcome
Among several risk scores predicting short-term or mid-term risk of ischaemic events, the Global Registry of Acute Coronary Events (GRACE) and the TIMI risk scores are the most widely used. There are some differences with respect to populations, outcomes, and time frames, as well as predictors derived from baseline characteristics, history, clinical or haemodynamic presentation, ECG, laboratory measures, and treatment.
Based on direct comparisons, the GRACE risk score provides the most accurate stratification of risk both on admission and at discharge due to its good discriminative power (Table 3). However, the complexity of the estimation requires the use of computer or personal digital assistant software for risk calculations, which can also be performed online (http://www.outcomes.org/grace).
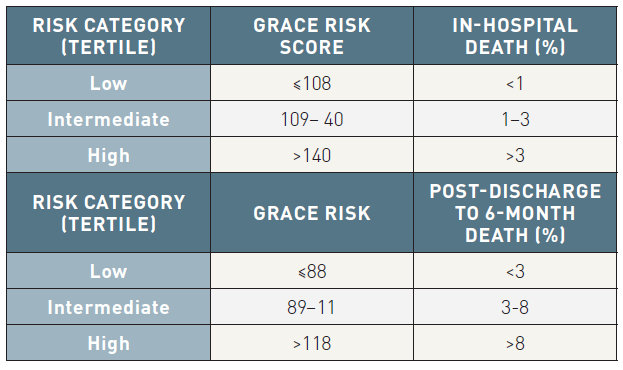
Table 3
Bleeding risk scores
Bleeding is associated with an adverse prognosis in NSTE-ACS and all efforts should be made to reduce bleeding whenever possible. A few variables can help to classify patients into different levels of risk for major bleeding during hospitalisation. Bleeding risk scores have been developed from registry or trial cohorts in the setting of ACS and PCI. The Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines (CRUSADE) bleeding risk score (www.crusadebleedingscore.org/) was developed from a cohort of patients from the CRUSADE registry (derivation cohort) and further validated in a validation cohort (Table 4) . The rate of major bleeding increased gradually with rising bleeding risk score (Figure 2). The risk score was developed from cohorts where femoral access was predominately or exclusively used. Its predictive value may be lower in a radial access setting.
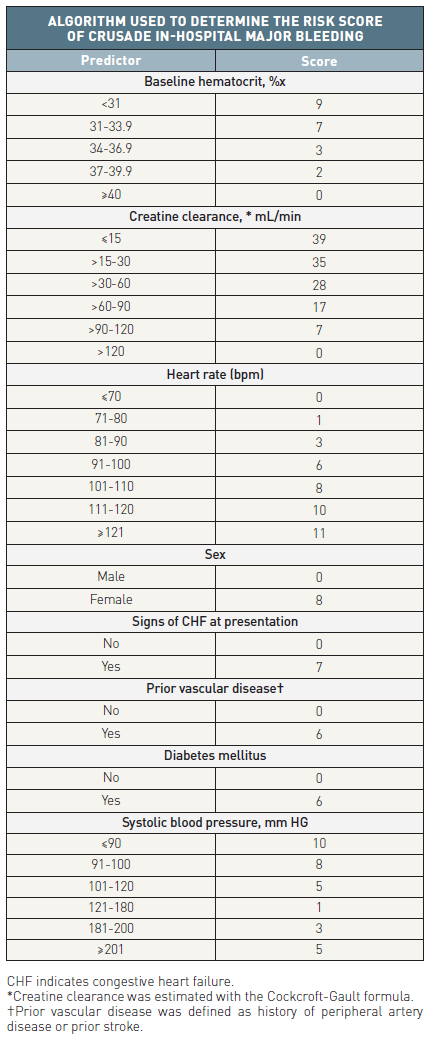
Table 4
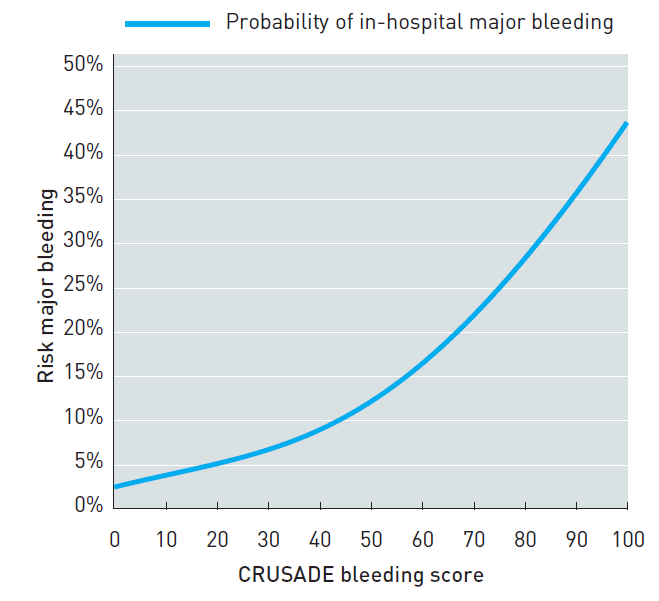
Figure 2
Treatment
ANTI-ISCHAEMIC AGENTS
Anti-ischaemic drugs either decrease myocardial oxygen demand (by decreasing heart rate, lowering blood pressure, reducing preload or reducing myocardial contractility) or increase myocardial oxygen supply (by inducing coronary vasodilatation).
ANTIPLATELET AGENTS
Platelet activation and subsequent aggregation play a dominant role in the propagation of arterial thrombosis and consequently are the key therapeutic targets in the management of ACS. Antiplatelet therapy should be instituted as early as possible when the diagnosis of NSTE-ACS is made in order to reduce the risk of both acute ischaemic complications and recurrent atherothrombotic events. Platelets can be inhibited by three classes of drugs, each of which has a distinct mechanism of action.
Aspirin (acetyl salicylic acid)
Based on studies performed 30 years ago, aspirin reduces the incidence of recurrent MI or death in patients with what was then called unstable angina (odds ratio [OR] 0.47; CI: 0.37-0.61; p<0.001) . A loading dose of chewed, plain aspirin between 150 and 300 mg is recommended. Intravenous aspirin is an alternative mode of application, but has not been investigated in trials and is not available everywhere. A daily maintenance dose of 75-100 mg has the same efficacy as higher doses and carries a lower risk of gastrointestinal intolerance, which may require drug discontinuation in up to 1% of patients.
P2Y12 receptor inhibitors
An overview of the P2Y12 receptor inhibitors is given in Table 5A.
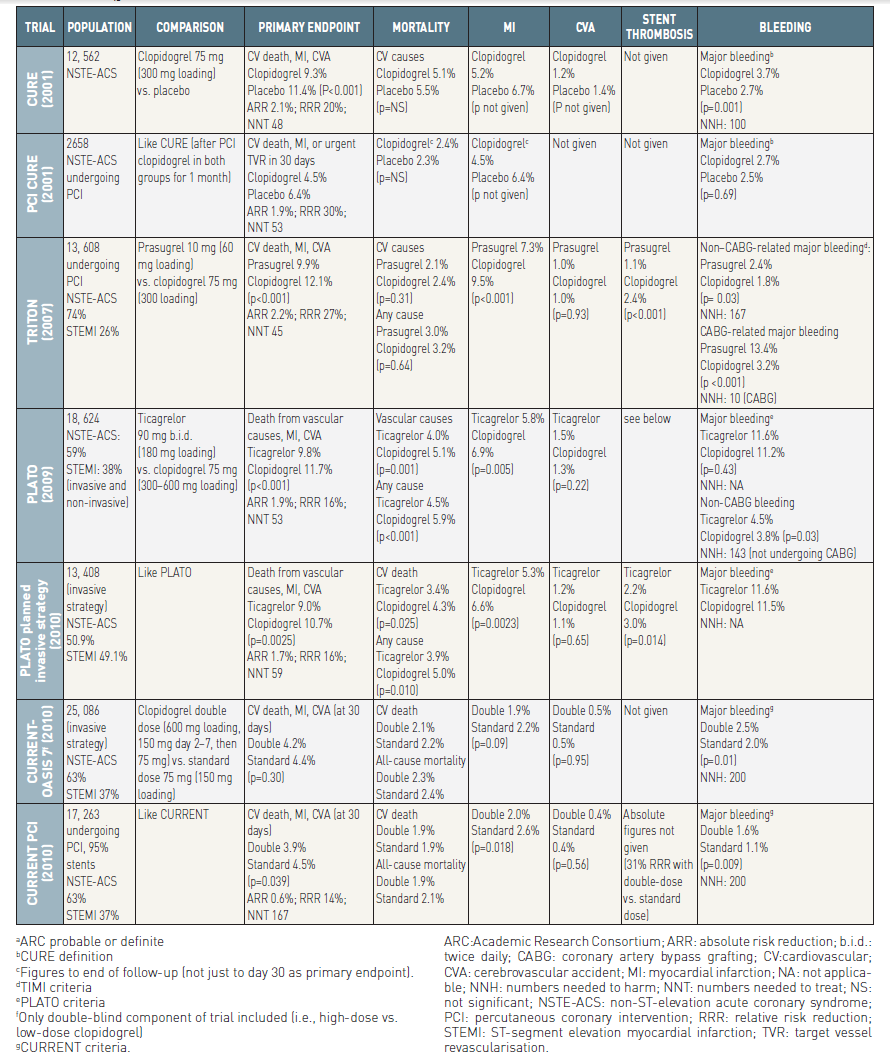
Table 5A
In the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial, a clopidogrel hydrogen sulphate 300 mg loading dose followed by 75 mg daily maintenance for 9-12 months in addition to aspirin reduced the incidence of cardiovascular death and non-fatal MI or stroke compared with aspirin alone (9.3% vs. 11.4%; RR 0.80; 95% CI: 0.72-0.90; p<0.001) in patients with NSTE-ACS associated with elevated cardiac markers or ST segment depression on ECG or age >60 years with prior CAD history .
An increase in the rate of major bleeding events was observed with clopidogrel (3.7% vs. 2.7%; RR 1.38; 95% CI: 1.13–1.67;p = 0.001), but with a non-significant increase in life-threatening and fatal bleeds . However, in the entire cohort, including patients submitted to revascularisation by either PCI or CABG, the benefit of clopidogrel treatment outweighed the risk of bleeding. Treating 1, 000 patients resulted in 21 fewer cardiovascular deaths, MIs, or strokes, at the cost of an excess of seven patients requiring transfusion and a trend for four patients to experience life-threatening bleeds.
The 600 mg loading dose of clopidogrel has a more rapid onset of action and more potent inhibitory effect than the 300 mg dose . There is wide variability in the pharmacodynamic response to clopidogrel linked to several factors, including genotype polymorphisms. Clopidogrel is converted to its active metabolite through two steps in the liver, which are dependent on cytochrome P450 (CYP) isoenzymes including CYP3A4 and CYP2C19.
Prasugrel
Prasugrel requires two metabolic steps for formation of its active metabolite, which is chemically similar to the active metabolite of clopidogrel . The first metabolic step requires only plasma esterases; the second step, in the liver, is the only step mediated by CYP enzymes. Consequently prasugrel produces more rapid and consistent platelet inhibition compared with clopidogrel . Response to prasugrel does not appear to be affected significantly by CYP inhibitors, including proton pump inhibitors, or loss-of-function variants of the CYP2C19 gene; nor is it affected by reduced ABCB1 function.
In the TRITON-TIMI 38 trial, a prasugrel 60 mg loading dose followed by 10 mg daily was compared with a clopidogrel 300 mg loading dose and then 75 mg daily in clopidogrel-naïve patients undergoing PCI, either primary PCI for STEMI or for recent STEMI or moderate-to-high risk NSTE-ACS once coronary angiography had been performed . The composite primary endpoint (cardiovascular death, non-fatal MI, or stroke) occurred in 11.2% of clopidogrel-treated patients and in 9.3% of prasugrel-treated patients (HR 0.82; 95% CI: 0.73–0.93; p= 0.002), mostly driven by a significant risk reduction for MI (9.2% to 7.1%; relative risk reduction [RRR] 23.9%; 95% CI: 12.7–33.7; p<0.001) . There was no difference in the rates of either non-fatal stroke or cardiovascular death. In the whole cohort, the rate of definite or probable stent thrombosis (as defined by the ARC) was significantly reduced in the prasugrel group compared with the clopidogrel group (1.1% vs. 2.4%, respectively; HR 0.48; 95% CI: 0.36–0.64; p<0.001). The corresponding figures for NSTE-ACS patients are not available. In the whole cohort, there was a significant increase in the rate of non–CABG-related TIMI major bleeding (2.4% vs. 1.8%; HR 1.32; 95% CI: 1.03–1.68; p= 0.03), mostly driven by a significant increase in spontaneous bleeds (1.6% vs. 1.1%; HR 1.51; 95% CI: 1.09–2.08; p= 0.01), but not by bleeding related to arterial access (0.7% vs. 0.6%; HR 1.18; 95% CI: 0.77–1.82; p= 0.45), which means that long-term exposure to a potent antiplatelet agent is the determinant of bleeding. Life-threatening bleeding was significantly increased under prasugrel with 1.4% vs. 0.9% (HR 1.52; 95% CI: 1.08–2.13; p= 0.01) as well as fatal bleeding with 0.4% vs. 0.1% (HR 4.19; 95% CI: 1.58–11.11; p= 0.002) with prasugrel compared to clopidogrel. There was evidence of net harm with prasugrel in patients with a history of cerebrovascular events [19+81]. In addition, there was no apparent net clinical benefit in patients over 75 years of age and in patients with low body weight (<60 kg). Greater benefit without increased risk of bleeding was observed in diabetic patients. There was no difference in efficacy in patients with (CrCl <60 mL/min) or without (CrCl >60 mL/min) renal impairment
In the recently published ISARREACT 5 trial a total 4,018 patients presented with acute coronary syndrome were randomly assigned to receive either ticagrelor or prasugrel . The primary end point was the composite of death, myocardial infarction, or stroke at 1 year. A major secondary end point (the safety end point) was bleeding. A primary-end point event occurred in 184 of 2012 patients (9.3%) in the ticagrelor group and in 137 of 2006 patients (6.9%) in the prasugrel group (hazard ratio, 1.36; P = 0.006). The respective incidences of the individual components of the primary end point in the ticagrelor group and the prasugrel group were as follows: death, 4.5% and 3.7%; myocardial infarction, 4.8% and 3.0%; and stroke, 1.1% and 1.0%. Definite or probable stent thrombosis occurred in 1.3% of patients assigned to ticagrelor and 1.0% of patients assigned to prasugrel, and definite stent thrombosis occurred in 1.1% and 0.6%, respectively. Major bleeding (as defined by the Bleeding Academic Research Consortium scale) was observed in 5.4% of patients in the ticagrelor group and in 4.8% of patients in the prasugrel group (hazard ratio, 1.12; 95% CI, 0.83 to 1.51; P = 0.46). Therefore, patients who presented with acute coronary syndromes with or without ST-segment elevation seem to higher benefit from a therapy with prasugrel in absence of any contraindications.
Ticagrelor
Ticagrelor belongs to a novel chemical class, cyclopentyl-triazolopyrimidine, and is an oral, reversibly binding P2Y12 inhibitor with a plasma half-life of approximately 12 hours. The level of P2Y12 inhibition is determined by the plasma ticagrelor level and, to a lesser extent, an active metabolite. Like prasugrel, it has a more rapid and consistent onset of action compared with clopidogrel, but additionally it has a quicker offset of action so that recovery of platelet function is faster (Table 5B) .
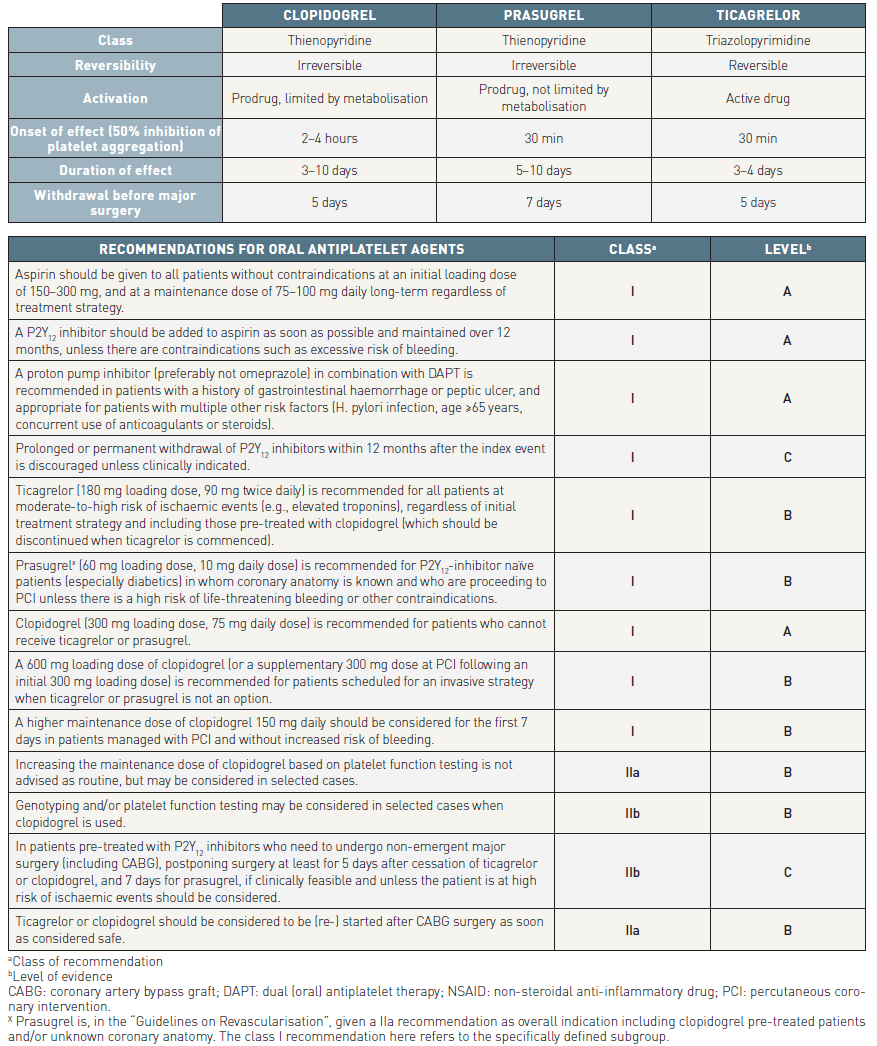
Table 5B
In the PLATelet inhibition and patient Outcomes (PLATO) trial, patients with either moderate-to-high risk NSTE-ACS (planned for either conservative or invasive management) or STEMI planned for primary PCI were randomised to either clopidogrel 75 mg daily, with a loading dose of 300 mg, or ticagrelor 180 mg loading dose followed by 90 mg twice daily . Patients undergoing PCI were allowed to receive an additional blinded 300 mg loading dose of clopidogrel clopidogrel (total loading dose 600 mg) or placebo and were also recommended to receive an additional 90 mg of ticagrelor (or placebo) if >24 h had elapsed since the initial loading dose. Treatment was continued for up to 12 months, with a minimum intended treatment duration of 6 months, and a median duration of study drug exposure of 9 months . In the overall cohort, the primary composite efficacy endpoint (death from vascular causes, MI, or stroke) was reduced with ticagrelor compared with clopidogrel [10.0% vs.12.3%; HR 0.83 (95% CI 0.74, 0.93), P = 0.0013], with similar reductions for cardiovascular death [3.7% vs. 4.9%; HR 0.77 (95% CI 0.64, 0.93), P = 0.007] and all-cause mortality [4.3% vs. 5.8%; HR 0.76 (95% CI 0.64, 0.90), P=0.002]. Differences in bleeding event rates were also similar in the NSTE-ACS subgroup compared with the overall study, with increased risk of non-CABG-related PLATO-defined major bleeds with ticagrelor compared with clopidogrel [4.8% vs.3.8%; HR 1.28 (95% CI 1.05, 1.56), P = 0.0139] but no difference in life-threatening or fatal bleeds. The benefits of ticagrelor compared with clopidogrel in NSTE-ACS were independent of whether or not revascularization was performed in the first 10 days after randomization .
The rate of definite stent thrombosis was reduced from 1.9% to 1.3% (p<0.01) and total mortality from 5.9% to 4.5% (p<0.001). Overall there was no significant difference in PLATO-defined major bleeding rates between the clopidogrel and ticagrelor groups (11.2% vs. 11.6%, respectively; p= 0.43). Major bleeding unrelated to CABG surgery was increased from 3.8% in the clopidogrel group to 4.5% in the ticagrelor group (HR 1.19; 95% CI: 1.02–1.38; p= 0.03).
In addition to increased rates of minor or non–CABG-related major bleeding with ticagrelor, adverse effects include dyspnoea, increased frequency of ventricular pauses, and asymptomatic increases in uric acid. The dyspnoea induced by ticagrelor occurs most frequently (up to 15%) within the first week of treatment and may be transient or persist until cessation of treatment, however it does not appear to be associated with any deterioration in cardiac or pulmonary function.
Nevertheless due to the results from the ISARREACT 5 trial, mentioned above, ticacrelor is a effective alternative, particularly in presence of any contraindications for prasugrel.
Prolonged dual antiplatelet therapy (DAPT)
Several studies suggest that DAPT continued after the first year results in the reduction of major adverse cardiovascular and cerebral events (MACCE). The prolonged DAPT trial included patients who were on DAPT including ASA and clopidogrel one year after stent implantation . Patients continued DAPT/placebo for a further 18 months. Overall, the trial revealed a significant reduction of acute stent thrombosis and MACCE, although a significantly higher bleeding rate and a higher overall mortality were identified. Further subgroup analysis revealed that the MACCE rate was only significantly reduced in patients with ACS. Interestingly, the rate of myocardial infarction and stent thrombosis was significantly reduced in both groups, patients with and without ACS . Prolonged DAPT that included ticagrelor was examined by the PEGASUS trial . A total of 21,162 patients who had had NSTEMI or STEMI within the last 3 years were randomized to either ticagrelor (60 mg or 90 mg twice daily) or placebo. After 3 years of therapy the combined primary endpoint of myocardial infarction and stroke was significantly reduced (1.1% vs. 1.2%; p=0.008). No differences were identified for cardiovascular death (p=0.06). Ticagrelor doses of 90 mg and 60 mg yielded similar results. Comparable to the DAPT trial, however, the bleeding risk (TIMI major) was at least doubled after prolonged ticagrelor therapy (90 mg: 2.6%, 60 mg: 2.3%; placebo 1.06%). Nevertheless, no significant differences were observed regarding fatal and intracranial bleeding events. These results were confirmed by a large meta-analysis including 33,435 patients after ACS with prolonged ticagrelor therapy . Due to a significantly higher rate of severe bleeding events, however, indication for prolonged DAPT should be restricted to patients with a high cardiovascular risk profile. Further subanalyses should be able to pinpoint which specific patient groups may profit from prolonged DAPT, including those with higher bleeding risk.
Prolonged dual antiplatelet therapy (DAPT)
- All patients with NSTE-ACS should receive either Prasugrel or Ticagrelor unless there are contraindications over at least 12 months
Shortened period of dual antiplatelet therapy (DAPT)
The greatest anti-ischaemic benefit of potent antiplatelet drugs occurs early, while most excess bleeding events arise during chronic treatment. Therefore, shortened period of DAPT may provide benefit especially in patient with high risk for bleeding complications.
In this context the TROPICAL-ACS trial randomized 2,610 if they had biomarker-positive acute coronary syndrome with successful PCI and a planned duration of dual antiplatelet treatment of 12 months . Enrolled patients were randomly assigned (1:1) to either standard treatment with prasugrel for 12 months (control group) or a step-down regimen (1 week prasugrel followed by 1 week clopidogrel and PFT-guided maintenance therapy with clopidogrel or prasugrel from day 14 after hospital discharge; guided de-escalation group). The primary endpoint was net clinical benefit (cardiovascular death, myocardial infarction, stroke or bleeding grade 2 or higher according to Bleeding Academic Research Consortium [BARC]) criteria) 1 year after randomisation. It occurred in 95 patients (7%) in the guided de-escalation group and in 118 patients (9%) in the control group (pnon-inferiority=0.0004; hazard ratio [HR] 0·81 [95% CI 0.62-1.06], p for superiority=0.12). Despite early de-escalation, there was no increase in the combined risk of cardiovascular death, myocardial infarction, or stroke in the de-escalation group (32 patients [3%]) versus in the control group (42 patients [3%]; p for non-inferiority=0.0115). Hence early de-escalation of antiplatelet treatment may be considered in selected patients with acute coronary syndrome managed with PCI and high risk of bleeding.
The Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention (TWILIGHT) trial was recently published . The randomised trial examined the effect of ticagrelor alone as compared with ticagrelor plus aspirin with regard to clinically relevant bleeding among patients who were at high risk for bleeding or an ischemic event and had undergone PCI. The primary end point was Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding. In the trial in total 9,006 patients were enrolled and 7,119 patients were randomised after 3 months. Nearly 30% of included patients received PCI due to NSTE-ACS. The incidence of the primary end point was 4.0% among patients randomly assigned to receive ticagrelor plus placebo and 7.1% among patients assigned to receive ticagrelor plus aspirin (hazard ratio, 0.56; 95% confidence interval [CI], 0.45 to 0.68; P<0.001). The difference in risk between the groups was similar for BARC type 3 or 5 bleeding (incidence, 1.0% among patients receiving ticagrelor plus placebo and 2.0% among patients receiving ticagrelor plus aspirin; hazard ratio, 0.49; 95% CI, 0.33 to 0.74). The incidence of death from any cause, nonfatal myocardial infarction, or nonfatal stroke was 3.9% in both groups (difference, −0.06 percentage points; 95% CI, −0.97 to 0.84; hazard ratio, 0.99; 95% CI, 0.78 to 1.25; P<0.001 for noninferiority).
The Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI were examined in the STOPDAPT-2 Randomized Clinical Trial . Among 3045 included 38% needed PCI due to NSTE-ACS. Patients were randomized either to 1 month of DAPT followed by clopidogrel monotherapy (n=1523) or to 12 months of DAPT with aspirin and clopidogrel (n=1522). One-month DAPT was both noninferior and superior to 12-month DAPT for the primary end point, occurring in 2.36% with 1-month DAPT and 3.70% with 12-month DAPT (absolute difference, −1.34% [95% CI, −2.57% to −0.11%]; hazard ratio [HR], 0.64 [95% CI, 0.42-0.98]), meeting criteria for noninferiority (P < 0.001) and for superiority (P = 0.04). The major secondary cardiovascular end point occurred in 1.96% with 1-month DAPT and 2.51% with 12-month DAPT (absolute difference, −0.55% [95% CI, −1.62% to 0.52%]; HR, 0.79 [95% CI, 0.49-1.29]), meeting criteria for noninferiority (P = 0.005) but not for superiority (P = 0.34). The major secondary bleeding end point occurred in 0.41% with 1-month DAPT and 1.54% with 12-month DAPT (absolute difference, −1.13% [95% CI, −1.84% to −0.42%]; HR, 0.26 [95% CI, 0.11-0.64]; P = 0.004 for superiority). The results have only limited clinical value, since Clopidogrel is only second choice P2Y12 inhibitor in ACS. However, the results confirm that aspirin can safely be stopped early in ACS without increasing the risk for ischaemic events.
Glycoprotein IIb/IIIa receptor inhibitors
The three GP IIb/IIIa receptor inhibitors approved for clinical use are intravenous (IV) agents belonging to different classes: abciximab is a monoclonal antibody fragment; eptifibatide is a cyclic peptide; and tirofiban is a peptidomimetic molecule. A meta-analysis of 29,570 patients initially medically managed and planned for PCI showed a moderate 9% RRR in death or non-fatal MI with GP IIb/IIIa receptor inhibitors (10.7% vs. 11.5%; p= 0.02) . No reduction in death or MI was seen in purely medically managed patients receiving GP IIb/IIIa receptor inhibitors versus placebo. The only significant benefit was observed when GP IIb/IIIa receptor inhibitors were maintained during PCI (10.5% vs. 13.6%; OR 0.74; 95% CI: 0.57–0.96; p= 0.02). The use of GP IIb/IIIa receptor inhibitors was associated with an increase in major bleeding complications, but intracranial bleeding was not significantly increased. Many of the older trials with these inhibitors were carried out in the absence of clopidogrel or newer P2Y12 inhibitors.
Upstream versus procedural initiation of GP IIb/IIIa receptor inhibitors
In the ACUITY Timing trial, deferred selective (only during PCI) versus routine upstream administration of any GP IIb/IIIa receptor inhibitor was tested among 9, 207 patients in a 2 x 2 factorial design . The net clinical outcome (incorporating both the ischaemic outcomes and major bleeding) at 30 days was similar (11.7% vs. 11.7%; RR 1.00; 95% CI: 0.89–1.11; p= 0.93; p-value for non-inferiority <0.001).
The Early Glycoprotein IIb/IIIa Inhibition in Non-ST-Segment Elevation Acute Coronary Syndrome (EARLY-ACS) trial randomised 9,492 patients assigned to an invasive strategy to early eptifibatide or placebo with provisional use of eptifibatide after angiography for PCI . The primary endpoint was a composite of death, MI, recurrent ischaemia requiring urgent revascularisation, or the occurrence of “thrombotic bail-out” (thrombotic complication during PCI that required the use of the bailout kit) at 96 hours. There was no significant reduction in the primary outcome in the early versus delayed provisional eptifibatide groups (9.3% vs. 10.0%; OR 0.92; 95% CI: 0.80–1.06; p= 0.23). Major bleeding rates were higher among patients assigned to early eptifibatide compared with delayed provisional therapy (TIMI major bleed at 120 h, 2.6% vs. 1.8%; OR 1.42; 95% CI: 1.97–1.89; p= 0.015). Accordingly, this trial demonstrated no advantage with a routine upstream use of eptifibatide in an invasive strategy compared to a delayed provisional strategy in the setting of contemporary antithrombotic therapy, where the minority of patients having PCI received eptifibatide in the delayed provisional arm.
Consistent across the trials is the signal for higher rates of bleeding with upstream GP IIb/IIIa treatment. Thus it is reasonable to withhold GP IIb/IIIa receptor inhibitors until after diagnostic angiography. In patients undergoing PCI their use can be based on angiographic results (e.g., presence of thrombus and extent of disease), troponin elevation, previous treatment with a P2Y12 inhibitor, patient age, and other factors influencing the risk of serious bleeding , . Upstream use of GP IIb/IIIa receptor inhibitors may be considered if there is active ongoing ischaemia among high-risk patients or where DAPT is not feasible. Patients who receive initial treatment with eptifibatide or tirofiban before angiography should be maintained on the same drug during and after PCI.
Comparative efficacy of GP IIb/IIIa receptor inhibitors
Abciximab was tested in the setting of PCI in a head-to-head comparison versus tirofiban in the TARGET trial, in which two-thirds of the patients had NSTE-ACS . Abciximab was shown to be superior to tirofiban in standard doses in reducing the risk of death, MI, and urgent revascularisation at 30 days, but the difference was not significant at 6 months. Further trials explored higher doses of tirofiban in various clinical settings and the results of meta-analyses suggest that high-dose bolus tirofiban (25 μg/kg followed by infusion) has similar efficacy to abciximab . There are no comparable data for eptifibatide.
Combination of GP IIb/IIIa receptor inhibitors with aspirin and a P2Y12 inhibitor
Limited data are available about the benefits of adding a GP IIb/IIIa receptor inhibitor to the combination of aspirin with a P2Y12 inhibitor in the setting of NSTE-ACS. In the Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment-2 (ISAR-REACT-2) trial, 2, 022 high-risk NSTE-ACS patients were randomised following pretreatment with aspirin and 600 mg of clopidogrel to either abciximab or placebo during PCI. The 30-day composite endpoint of death, MI, or urgent target vessel revascularisation occurred significantly less frequently in abciximab-treated patients versus placebo (8.9% vs. 11.9%; RR 0.75; 95% CI: 0.58–0.97; p= 0.03). Most of the risk reduction with abciximab resulted from a reduction in death and non-fatal MI.
In the TRITON and PLATO trials the rates of use of GP IIb/IIIa receptor inhibitors were 55% and 27%, respectively. Patients receiving a GP IIb/IIIa receptor inhibitor in the TRITON trial had higher rates of TIMI major and minor non-CABG bleeding, but use of a GP IIb/IIIa receptor inhibitor did not influence the relative risk of bleeding with prasugrel compared to clopidogrel (p-value for interaction 0.19).
Prasugrel reduced rates of death, MI, or stroke compared to clopidogrel, both with (6.5% vs. 8.5%; HR 0.76; 95% CI: 0.64–0.90) and without (4.8% vs. 6.1%; HR 0.78; 95% CI: 0.63–0.97) GP IIb/IIIa receptor inhibitors. In the PLATO trial, ticagrelor also reduced rates of death, MI, or stroke in patients receiving (10.0% vs. 11.1%; HR 0.90; 95% CI: 0.76–1.07) or not receiving (9.7% vs. 11.9%; HR 0.82; 95% CI: 0.74–0.92) a GP IIb/IIIa receptor inhibitor .
Overall, it is reasonable to reserve combination therapy with a GP IIb/IIIa receptor inhibitor in addition toaspirin and a P2Y12 inhibitor for patients with NSTE-ACS undergoing PCI that are deemed to have a high risk of procedural MI and an absence of high risk for bleeding.
Glycoprotein IIb/IIIa inhibitors and adjunctive anticoagulant therapy
Most trials showing benefits of GP IIb/IIIa receptor inhibitors used an anticoagulant. Several trials in the field of NSTE-ACS, as well as observational studies in PCI, have shown that LMWH, predominantly enoxaparin, can be safely used with GP IIb/IIIa receptor inhibitors without compromising efficacy, although subcutaneous enoxaparin alone does not adequately protect against catheter thrombosis during primary PCI, despite this combination. In the Fifth Organization to Assess Strategies in Acute Ischemic Syndromes (OASIS-5) trial, GP IIb/IIIa receptor inhibitors were used with aspirin, clopidogrel, and either fondaparinux or enoxaparin . Overall, bleeding complications were lower with fondaparinux than with enoxaparin. Bivalirudin and unfractionated heparin [UFH]/LMWH were shown to have equivalent safety and efficacy when used with aspirin, clopidogrel, and a GP IIb/IIIa receptor inhibitor in the ACUITY trial . The combination of bivalirudin and a GP IIb/IIIa receptor inhibitor results in a similar rate of ischaemic events compared with bivalirudin alone, but is associated with a higher rate of major of bleeding events . Thus, this combination cannot be recommended for routine use.
Glycoprotein IIb/IIIa receptor inhibitors
The three GP IIb/IIIa receptor inhibitors approved for clinical use are intravenous (IV) agents belonging to different classes: abciximab is a monoclonal antibody fragment; eptifibatide is a cyclic peptide; and tirofiban is a peptidomimetic molecule. A meta-analysis of 29,570 patients initially medically managed and planned for PCI showed a moderate 9% RRR in death or non-fatal MI with GP IIb/IIIa receptor inhibitors (10.7% vs. 11.5%; p= 0.02) . No reduction in death or MI was seen in purely medically managed patients receiving GP IIb/IIIa receptor inhibitors versus placebo. The only significant benefit was observed when GP IIb/IIIa receptor inhibitors were maintained during PCI (10.5% vs. 13.6%; OR 0.74; 95% CI: 0.57–0.96; p= 0.02). The use of GP IIb/IIIa receptor inhibitors was associated with an increase in major bleeding complications, but intracranial bleeding was not significantly increased. Many of the older trials with these inhibitors were carried out in the absence of clopidogrel or newer P2Y12 inhibitors.
Upstream versus procedural initiation of GP IIb/IIIa receptor inhibitors
In the ACUITY Timing trial, deferred selective (only during PCI) versus routine upstream administration of any GP IIb/IIIa receptor inhibitor was tested among 9, 207 patients in a 2 x 2 factorial design . The net clinical outcome (incorporating both the ischaemic outcomes and major bleeding) at 30 days was similar (11.7% vs. 11.7%; RR 1.00; 95% CI: 0.89–1.11; p= 0.93; p-value for non-inferiority <0.001).
The Early Glycoprotein IIb/IIIa Inhibition in Non-ST-Segment Elevation Acute Coronary Syndrome (EARLY-ACS) trial randomised 9,492 patients assigned to an invasive strategy to early eptifibatide or placebo with provisional use of eptifibatide after angiography for PCI . The primary endpoint was a composite of death, MI, recurrent ischaemia requiring urgent revascularisation, or the occurrence of “thrombotic bail-out” (thrombotic complication during PCI that required the use of the bailout kit) at 96 hours. There was no significant reduction in the primary outcome in the early versus delayed provisional eptifibatide groups (9.3% vs. 10.0%; OR 0.92; 95% CI: 0.80–1.06; p= 0.23). Major bleeding rates were higher among patients assigned to early eptifibatide compared with delayed provisional therapy (TIMI major bleed at 120 h, 2.6% vs. 1.8%; OR 1.42; 95% CI: 1.97–1.89; p= 0.015). Accordingly, this trial demonstrated no advantage with a routine upstream use of eptifibatide in an invasive strategy compared to a delayed provisional strategy in the setting of contemporary antithrombotic therapy, where the minority of patients having PCI received eptifibatide in the delayed provisional arm.
Consistent across the trials is the signal for higher rates of bleeding with upstream GP IIb/IIIa treatment. Thus it is reasonable to withhold GP IIb/IIIa receptor inhibitors until after diagnostic angiography. In patients undergoing PCI their use can be based on angiographic results (e.g., presence of thrombus and extent of disease), troponin elevation, previous treatment with a P2Y12 inhibitor, patient age, and other factors influencing the risk of serious bleeding , . Upstream use of GP IIb/IIIa receptor inhibitors may be considered if there is active ongoing ischaemia among high-risk patients or where DAPT is not feasible. Patients who receive initial treatment with eptifibatide or tirofiban before angiography should be maintained on the same drug during and after PCI.
Comparative efficacy of GP IIb/IIIa receptor inhibitors
Abciximab was tested in the setting of PCI in a head-to-head comparison versus tirofiban in the TARGET trial, in which two-thirds of the patients had NSTE-ACS . Abciximab was shown to be superior to tirofiban in standard doses in reducing the risk of death, MI, and urgent revascularisation at 30 days, but the difference was not significant at 6 months. Further trials explored higher doses of tirofiban in various clinical settings and the results of meta-analyses suggest that high-dose bolus tirofiban (25 μg/kg followed by infusion) has similar efficacy to abciximab . There are no comparable data for eptifibatide.
Combination of GP IIb/IIIa receptor inhibitors with aspirin and a P2Y12 inhibitor
Limited data are available about the benefits of adding a GP IIb/IIIa receptor inhibitor to the combination of aspirin with a P2Y12 inhibitor in the setting of NSTE-ACS. In the Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment-2 (ISAR-REACT-2) trial, 2, 022 high-risk NSTE-ACS patients were randomised following pretreatment with aspirin and 600 mg of clopidogrel to either abciximab or placebo during PCI. The 30-day composite endpoint of death, MI, or urgent target vessel revascularisation occurred significantly less frequently in abciximab-treated patients versus placebo (8.9% vs. 11.9%; RR 0.75; 95% CI: 0.58–0.97; p= 0.03). Most of the risk reduction with abciximab resulted from a reduction in death and non-fatal MI.
In the TRITON and PLATO trials the rates of use of GP IIb/IIIa receptor inhibitors were 55% and 27%, respectively. Patients receiving a GP IIb/IIIa receptor inhibitor in the TRITON trial had higher rates of TIMI major and minor non-CABG bleeding, but use of a GP IIb/IIIa receptor inhibitor did not influence the relative risk of bleeding with prasugrel compared to clopidogrel (p-value for interaction 0.19).
Prasugrel reduced rates of death, MI, or stroke compared to clopidogrel, both with (6.5% vs. 8.5%; HR 0.76; 95% CI: 0.64–0.90) and without (4.8% vs. 6.1%; HR 0.78; 95% CI: 0.63–0.97) GP IIb/IIIa receptor inhibitors. In the PLATO trial, ticagrelor also reduced rates of death, MI, or stroke in patients receiving (10.0% vs. 11.1%; HR 0.90; 95% CI: 0.76–1.07) or not receiving (9.7% vs. 11.9%; HR 0.82; 95% CI: 0.74–0.92) a GP IIb/IIIa receptor inhibitor .
Overall, it is reasonable to reserve combination therapy with a GP IIb/IIIa receptor inhibitor in addition to aspirin and a P2Y12 inhibitor for patients with NSTE-ACS undergoing PCI that are deemed to have a high risk of procedural MI and an absence of high risk for bleeding.
Glycoprotein IIb/IIIa inhibitors and adjunctive anticoagulant therapy
Most trials showing benefits of GP IIb/IIIa receptor inhibitors used an anticoagulant. Several trials in the field of NSTE-ACS, as well as observational studies in PCI, have shown that LMWH, predominantly enoxaparin, can be safely used with GP IIb/IIIa receptor inhibitors without compromising efficacy, although subcutaneous enoxaparin alone does not adequately protect against catheter thrombosis during primary PCI, despite this combination. In the Fifth Organization to Assess Strategies in Acute Ischemic Syndromes (OASIS-5) trial, GP IIb/IIIa receptor inhibitors were used with aspirin, clopidogrel, and either fondaparinux or enoxaparin . Overall, bleeding complications were lower with fondaparinux than with enoxaparin. Bivalirudin and unfractionated heparin [UFH]/LMWH were shown to have equivalent safety and efficacy when used with aspirin, clopidogrel, and a GP IIb/IIIa receptor inhibitor in the ACUITY trial . The combination of bivalirudin and a GP IIb/IIIa receptor inhibitor results in a similar rate of ischaemic events compared with bivalirudin alone, but is associated with a higher rate of major of bleeding events . Thus, this combination cannot be recommended for routine use.
Glycoprotein IIb/IIIa receptor inhibitors and CABG
Patients undergoing CABG surgery whilst receiving GP IIb/IIIa receptor inhibitors require appropriate measures to ensure adequate haemostasis and discontinuation of GP IIb/IIIa receptor inhibitors before or, if not feasible, at the time of surgery. Eptifibatide and tirofiban have a short half-life (approximately 2 hours), so platelet function due to reversible receptor binding can recover by the end of CABG surgery. Abciximab has a short plasma half-life (10 min) but dissociates slowly from the platelet, with a half-life of approximately 4 hours, so that recovery of platelet aggregation responses to normal or near-normal takes approximately 48 hours after the infusion has been terminated (although receptor-bound abciximab can be detected for much longer).
ANTICOAGULANTS
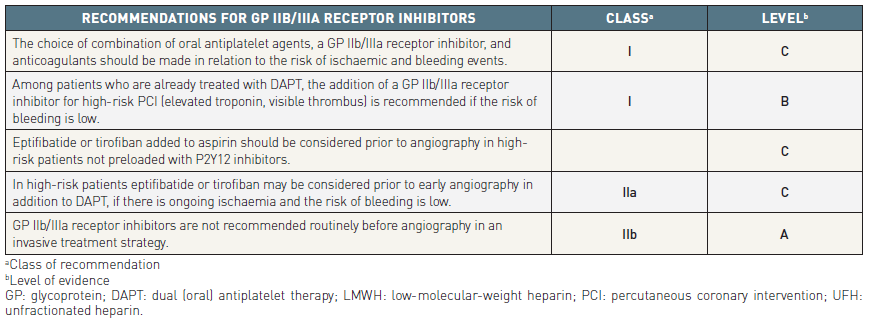
Table 6
Anticoagulants are used in the treatment of NSTE-ACS to inhibit thrombin generation and/or activity, thereby reducing thrombus-related events. There is evidence that anticoagulation is effective in addition to platelet inhibition and that the combination of the two is more effective than either treatment alone. Several anticoagulants, which act at different levels of the coagulation cascade, have been investigated or are under investigation in NSTE-ACS. For a review of anticoagulants and their action on the coagulation cascade see (Figure 3).
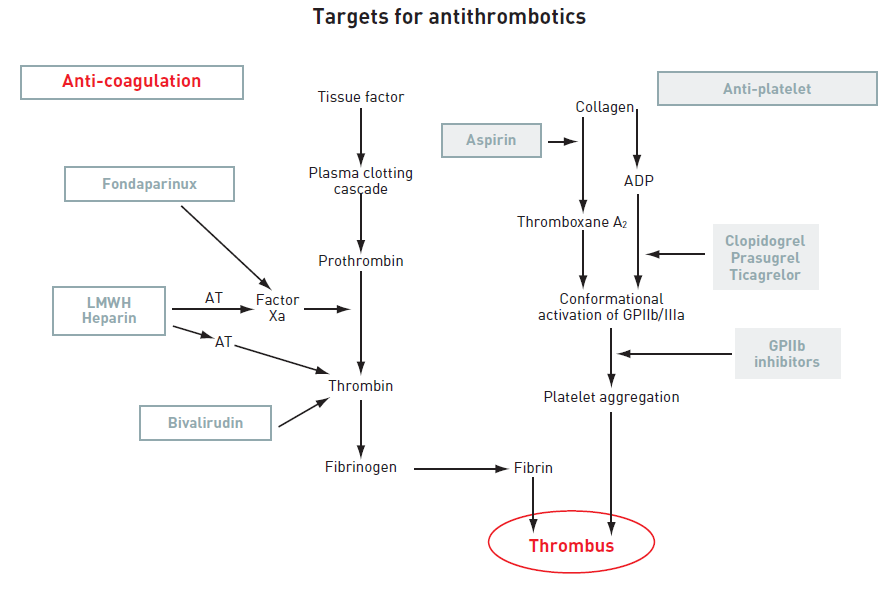
Figure 3
ADP: adenosine diphosphate; AT: antithrombin; GP: glycoprotein; LMWH: low-molecular-weight heparin; TRA: thrombin receptor antagonist.
Indirect inhibitors of the coagulation cascade
Fondaparinux
The only selective factor-Xa inhibitor available for clinical use is fondaparinux. It inhibits coagulation factor Xa by binding reversibly and non-covalently to antithrombin, with a high affinity. Fondaparinux increases 300-fold the ability of antithrombin to inhibit factor Xa.
Fondaparinux has 100% bioavailability after subcutaneous injection, with an elimination half-life of 17 hours, and can therefore be given once daily. It is eliminated mainly by the kidneys, and is contraindicated if CrCl is <20 mL/min. No definite case of heparin-induced thrombocytopenia (HIT) has been reported with this drug. Therefore monitoring of platelet count is not necessary. No dose adjustment and no monitoring of anti-Xa activity are required.
In ACS, a 2.5 mg fixed daily dose of fondaparinux is recommended. This dose was selected on the basis of two large phase III trials (OASIS-5 and OASIS-6) , .
In the OASIS-5 study, 20, 078 patients with NSTE-ACS were randomised to receive 2.5 mg of subcutaneous fondaparinux once daily or subcutaneous enoxaparin 1 mg/kg twice daily for 5 days on average . The primary efficacy outcome of death, MI, or refractory ischaemia at 9 days was 5.7% for enoxaparin versus 5.8% for fondaparinux (HR 1.01; 95% CI: 0.90–1.13), fulfilling the criteria for non-inferiority. At the same point, major bleedings were halved with fondaparinux: 2.2% compared to 4.1% with enoxaparin (HR 0.52; 95% CI: 0.44–0.61; p<0.001). Major bleeding was an independent predictor of long-term mortality, which was significantly reduced with fondaparinux at 30 days (2.9% vs. 3.5%; HR 0.83; 95% CI: 0.71–0.97; p= 0.02) and at 6 months (5.8% vs. 6.5%; HR 0.89; 95% CI: 0.80–1.00; p= 0.05). At 6 months the composite endpoint of death, MI, or stroke was significantly lower with fondaparinux versus enoxaparin (11.3% vs. 12.5%; HR 0.89; 95% CI: 0.82–0.97;p = 0.007). In the population submitted to PCI, a significantly lower rate of major bleeding complications (including access site complications) was observed at 9 days in the fondaparinux group versus enoxaparin (2.4% vs. 5.1%; HR 0.46; 95% CI: 0.35–0.61; p<0.001). Catheter thrombus was observed more frequently with fondaparinux (0.9%) than with enoxaparin (0.4%), but was abolished by injection of an empirically determined bolus of UFH at the time of PCI. As the rate of ischaemic events was similar in both the fondaparinux and heparin groups at 9 days, the net clinical benefit of death, MI, stroke, and major bleeding favoured fondaparinux versus enoxaparin (8.2% vs. 10.4%; HR 0.78; 95% CI: 0.67–0.93; p= 0.004).
Results from a large-scale Scandinavian registry exploring the uptake of fondaparinux compared with LMWH among 40 616 NSTEMI patients described a reduction in in-hospital mortality [OR 0.75 (95% CI 0.63, 0.89)] and bleeding events [OR 0.54 (95% CI 0.42, 0.70)] associated with the use of fondaparinux, but the advantage disappeared at 30 days and 6 months, respectively . In summary, fondaparinux is considered to be the parenteral anticoagulant with the most favourable therapeutic window and is recommended regardless of the management strategy unless the patient is scheduled for immediate coronary angiography.
Low-molecular weight heparins
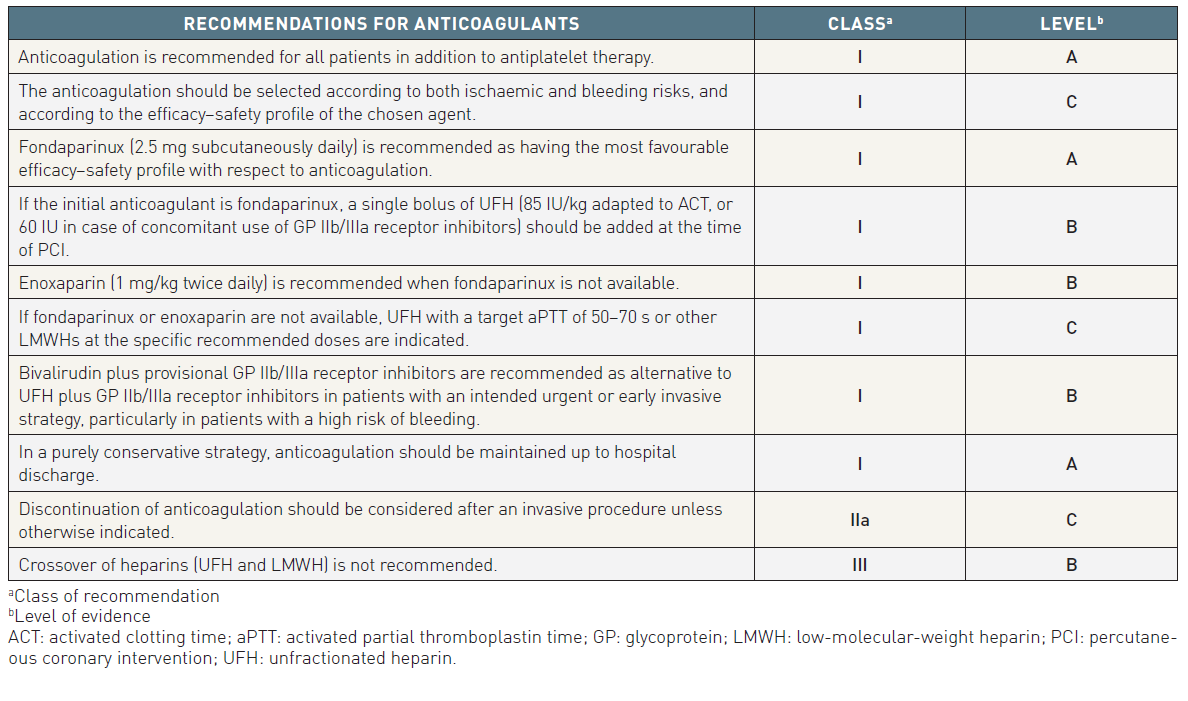
Table 7
Low-molecular weight heparins are a class of heparin-derived anti-Xa and anti-IIa compounds with molecular weights ranging from 2, 000 to 10, 000 Daltons. LMWHs have different pharmacokinetic properties and anticoagulant activities and are not therefore clinically interchangeable. LMWHs have several advantages over UFH, particularly an almost complete absorption after subcutaneous administration, less protein binding, less platelet activation and, thereby, a more predictable dose-effect relationship. Furthermore, there is a lower risk of HIT with LMWHs compared to UFH. LMWHs are eliminated at least partially by the renal route. The risk of accumulation increases with declining renal function, resulting in an increased bleeding risk. Most LMWHs are contraindicated in the case of renal failure with CrCl <30 mL/min. However, for enoxaparin, dose adaptation is advocated in patients with a CrCl <30 mL/min (1 mg/kg once instead of twice daily).
With the current doses used in clinical practice, monitoring of anti-Xa activity is not necessary, except in special populations of patients such as those with renal failure or obesity.
Several meta-analyses have been published concerning the relative efficacy of LMWHs versus UFH in NSTE-ACS. The first, which included 12 trials with different drugs totalling 17, 157 patients, confirmed that heparins in aspirin-treated NSTE-ACS patients conferred a significant benefit over placebo in terms of death or MI (OR 0.53; 95% CI: 0.38–0.73; p=0.0001). There was no significant advantage in favour of LMWHs compared to UFH with regard to efficacy or safety endpoints . A meta-analysis of all trials testing enoxaparin versus UFH, totalling 21, 946 patients, showed no significant difference between the two compounds for death at 30 days (3.0% vs. 3.0%; OR 1.00; 95% CI: 0.85–1.17; p=not significant). A significant reduction in the combined endpoint of death or MI at 30 days was observed in favour of enoxaparin versus UFH (10.1% vs. 11.0%; OR 0.91; 95% CI: 0.83–0.99).
The respective efficacy and safety of LMWHs compared with UFH when prescribed in association with GP IIb/IIIa receptor inhibitors was explored in a number of small-scale trials. Overall there was no significant difference in safety endpoints. These trials failed to show a difference in efficacy in terms of “hard” endpoints, except in the Integrilin and Enoxaparin Randomized Assessment of Acute Coronary Syndrome Treatment (INTERACT) trial, where a significant difference in favour of enoxaparin plus eptifibatide was observed over UFH plus eptifibatide . However, none of these trials had sufficient statistical power to draw definitive conclusions.
Most of these trials were carried out at a time when an invasive strategy was not routine practice, and in some an invasive strategy was not encouraged. As a result only a minority of patients in these trials underwent invasive treatment, and any conclusions that may be drawn from these studies are now likely to be considered outdated.
The only trial to test enoxaparin versus UFH using a contemporary approach, with a high rate of PCI, revascularisation, stent implantation, and active antiplatelet therapy with aspirin, clopidogrel, and GP IIb/IIIa receptor inhibitors, was the Superior Yield of the New Strategy of Enoxaparin, Revascularization and Glycoprotein IIb/IIIa Inhibitors (SYNERGY) trial . This trial included 10, 027 high-risk patients undergoing early invasive evaluation plus revascularisation, of which 76% received anticoagulants prior to randomisation. No significant difference was observed in terms of death and MI at 30 days (enoxaparin vs. UFH, 14.0% vs. 14.5%; OR 0.96; 95% CI: 0.86–1.06; p=not significant). More bleeding events occurred with enoxaparin, with a statistically significant increase in TIMI major bleeding (9.1% vs. 7.6%; p=0.008).
In NSTE-ACS patients pre-treated with enoxaparin, no additional enoxaparin is recommended during PCI if the last subcutaneous enoxaparin injection was administered <8 hours before PCI, whereas an additional 0.3 mg/kg IV bolus is recommended if the last subcutaneous enoxaparin injection was administered >8 hours before PCI. Crossing over to another anticoagulant during PCI is strongly discouraged.
Unfractionated heparin
Unfractionated heparin is a heterogeneous mixture of polysaccharide molecules, with a molecular weight ranging from 2, 000 to 30, 000 (mostly 15–18, 000) Daltons. UFH is poorly absorbed by the subcutaneous route, so IV infusion is the preferred route of administration. The therapeutic window is narrow, requiring frequent monitoring of aPTT, with an optimal target level of 50–75 s, corresponding to 1.5–2.5 times the upper limit of normal. At higher aPTT values, the risk of bleeding complications is increased, without further antithrombotic benefits. A weight-adjusted dose of UFH is recommended, at an initial bolus of 60–70 IU/kg with a maximum of 5000 IU, followed by an initial infusion of 12–15 IU/kg/h, to a maximum of 1, 000 IU/h. The anticoagulant effect of UFH is lost rapidly within a few hours after interruption.
A pooled analysis of six trials testing short-term UFH versus placebo or untreated controls showed a 33% risk reduction in death and MI (OR 0.67; 95% CI: 0.45–0.99; p=0.04) . The risk reduction for MI accounted for practically all of the beneficial effect. In trials comparing the combination of UFH plus aspirin versus aspirin alone in NSTE-ACS, a trend towards a benefit was observed in favour of the UFH–aspirin combination, but at the cost of an increased risk of bleeding. Recurrence of events after interruption of UFH explains why this benefit is not maintained over time, unless the patient is revascularised before the interruption of UFH.
In the PCI setting, UFH is given as an IV bolus either under ACT guidance (ACT in the range of 250–350 s, or 200–250 s if a GP IIb/IIIa receptor inhibitor is given) or in a weight-adjusted manner (usually 70–100 IU/kg or 50–60 IU/kg in combination with GP IIb/IIIa receptor inhibitors). Continued heparinisation after completion of the procedure, either preceding or following arterial sheath removal, is not recommended.
If the patient is taken to the catheterisation laboratory with an ongoing IV infusion of heparin, a further IV bolus of UFH should be adapted according to the ACT values and use of GP IIb/IIIa receptor inhibitors.
Direct thrombin inhibitors (bivalirudin)
Several DTIs have been tested over time, but only bivalirudin reached clinical use in PCI and ACS settings. Bivalirudin binds directly to thrombin (factor IIa) and thereby inhibits the thrombin-induced conversion of fibrinogen to fibrin. It inactivates fibrin-bound as well as fluid-phase thrombin. As it does not bind to plasma proteins, the anticoagulant effect is more predictable. Bivalirudin is eliminated via the kidneys. Coagulation tests (aPTT, ACT) correlate well with plasma concentrations.
Bivalirudin was initially tested in the setting of PCI. In the REPLACE-2 trial, bivalirudin plus provisional GP IIb/IIIa receptor inhibitors was shown to be non-inferior to UFH plus GP IIb/IIIa receptor inhibitors regarding protection against ischaemic events during PCI procedures, but with a significantly lower rate of major bleeding complications (2.4% vs. 4.1%, p<0.001) for bivalirudin. No significant difference was observed in the hard endpoints at 1 month, 6 months and 1 year. Bivalirudin is currently approved for urgent and elective PCI at a dose of 0.75 mg/kg bolus followed by 1.75 mg/kg/h. In NSTE-ACS patients, bivalirudin is recommended at a dose of 0.1 mg/kg IV bolus followed by an infusion of 0.25 mg/kg/h until PCI.
The ACUITY trial tested bivalirudin in the setting of NSTE-ACS and planned invasive strategy . Patients were randomised to one of three unblinded treatment groups: standard combination treatment with either UFH or LMWH with a GP IIb/IIIa receptor inhibitor (control arm) (n=4, 603); bivalirudin with a GP IIb/IIIa receptor inhibitor (n=4, 604); or bivalirudin alone (n=4, 612). Bivalirudin was started before angiography and was stopped after PCI. There was no significant difference between standard UFH/LMWHs plus GP IIb/IIIa receptor inhibitors, and the combination of bivalirudin and GP IIb/IIIa receptor inhibitors, for the composite ischaemia endpoint at 30 days (7.3% vs. 7.7%, respectively; RR 1.07; 95% CI: 0.92–1.23; p=0.39) or for major bleeding (5.7% vs. 5.3%; RR 0.93; 95% CI: 0.78–1.10; p=0.38). Bivalirudin alone was non-inferior to the standard UFH/LMWHs combined with GP IIb/IIIa receptor inhibitors with respect to the composite ischaemia endpoint (7.8% vs. 7.3%; RR 1.08; 95% CI: 0.93–1.24; p=0.32), but with a significantly lower rate of major bleeding (3.0% vs. 5.7%; RR 0.53; 95% CI: 0.43–0.65; p<0.001). Therefore the 30-day net clinical outcome was significantly better (10.1% vs. 11.7%; RR 0.86; 95% CI: 0.77–0.94; p=0.02) with bivalirudin alone versus UFH/LMWHs plus GP IIb/IIIa receptor inhibitors .
Recently, the MATRIX trial randomized 7213 patients with ACS for whom PCI was anticipated to either UFH or bivalirudin . The MACCE rate was not significantly lower with bivalirudin than with UFH (10.3% vs, 10.9%; p=0.44). In addition, the rates of net adverse events including bleeding events were comparable after bivalirudin and UFH (11.2% vs. 12.45; p=0.12). Even post-PCI infusion of bivalrudin, as compared with no infusion, did not significantly reduce net clinical adverse events or acute stent thrombosis (11.0% vs. 11.9%; p=0.34).
New anticoagulants
Two newer anticoagulants in addition to standard antiplatelet therapy were recently investigated in the setting of ACS in patients without indication for oral anticoagulation. The direct factor Xa-inhibitors apixaban and rivaroxaban have been tested in phase II and III-studies. APPRAISE-2 (apixaban) was stopped prematurely due to excessive bleeding with the apixaban regimen. The ATLAS-ACS2-TIMI51 trial (rivaroxaban) has shown a significant 16% reduction in the primary efficacy endpoint, which was a composite of cardiovascular death, myocardial infarction and stroke. Despite the fact that all-cause mortality could be reduced by 36% it has to be considered, that patients were treated with aspirin and clopidogrel and not with the more contemporary combination of aspirin and prasugrel or ticagrelor. Therefore, the findings are currently difficult to translate into the clinical routine.
Combination of anticoagulation and antiplatelet treatment
Anticoagulation and DAPT with aspirin and a P2Y12 inhibitor are recommended as first-line treatment during the initial phase of NSTE-ACS. The duration of anticoagulation is limited to the acute phase, whereas DAPT is recommended for 12 months with or without PCI and stent implantation. A sizeable proportion of patients (6%–8%) presenting with NSTE-ACS have an indication for long-term oral anticoagulation with a vitamin-K antagonist (VKA) due to various conditions such as moderate-to-high-embolic-risk atrial fibrillation, mechanical heart valves, or venous thromboembolism. Coronary angiography should be performed with oral anticoagulation (OAC), because interruption of OAC and bridging with parenteral anticoagulants may lead to an increase in both thromboembolic episodes and bleeds .
The choice of stent type – newer-generation drug-eluting stent (DES) vs. bare-metal stent (BMS) – in patients requiring long-term anticoagulation is a matter of controversy in the setting of NSTE-ACS. Due to the absence of conclusive data, the decision should be based on individual aspects including estimated probability of subsequent target vessel revascularization (TVR) due to restenosis. In the ZEUS trial, 1606 patients with either high bleeding risk (52%), high thrombosis risk (17%), or low restenosis risk (31%) were randomized to implantation with either the zotarolimus-eluting DES (n=802) or a BMS (n=804) . At 1 year, major adverse cardiovascular events were lower for patients implanted with a zotarolimus-eluting stent than for those with a BMS [17.5% vs. 22.1%; HR 0.76 (95% CI 0.61, 0.95), P=0.011], results that were due to reductions in TVR [5.9% vs. 10.7%; HR 0.53 (95% CI 0.37, 0.75), P<0.001], MI [2.9% vs. 8.1%; HR 0.35 (95% CI 0.22, 0.56), P=0.001] and definite/probable stent thrombosis [2.0% vs. 4.1%; HR 0.48 (95% CI 0.27, 0.88), P=0.019]. The benefit of the zotarolimus-eluting stent over the BMS remained consistent across all pre-specified subgroups, and, in particular, no significant differences in any bleeding events between treatment groups were identified. This study suggests that a newer-generation DES may be preferred in patients who cannot tolerate long-term exposure to DAPT, such as those needing chronic OAC.
Even when a dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor is commonly used to reduce recurrent cardiovascular events in patients with ACS, irrespective of whether they are managed medically or with PCI, and oral anticoagulation is the standard of care to reduce the risk of stroke and systemic embolism in patients with AF. The combination of DAPT with anticoagulation is associated with a high risk of bleeding. Double antithrombotic therapy has been proposed as alternative to triple therapy and was shown to be superior in terms of bleeding in the open label WOEST trial comparing clopidogrel versus clopidogrel and aspirin among patients requiring oral anticoagulation with Vitamin K antagonists (VKA) . Furthermore a recent metaanalysis including 4 large randomized trials (n = 10 026; WOEST, PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS) could support the use of NOAC plus P2Y12 inhibitor as the preferred regimen post–percutaneous coronary intervention for these high-risk patients with AF , , , . The overall prevalence of ACS varied from 28% to 61%. A regimen of NOACs plus P2Y12 inhibitor was associated with less bleeding compared with VKAs plus DAPT. Strategies omitting aspirin caused less bleeding, including intracranial bleeding, without significant difference in MACE, compared with strategies including aspirin. Compared with a regimen of vitamin K antagonist (VKA) plus dual antiplatelet therapy (DAPT; P2Y 12 inhibitor plus aspirin), the odds ratios (ORs) for TIMI major bleeding were 0.58 (95% CI, 0.31-1.08) for VKA plus P2Y 12 inhibitor, 0.49 (95% CI, 0.30-0.82) for non-VKA oral anticoagulant (NOAC) plus P2Y 12 inhibitor, and 0.70 (95% CI, 0.38-1.23) for NOAC plus DAPT. Compared with VKA plus DAPT, the ORs for MACE were 0.96 (95% CI, 0.60-1.46) for VKA plus P2Y 12 inhibitor, 1.02 (95% CI, 0.71-1.47) for NOAC plus P2Y 12 inhibitor, and 0.94 (95% CI, 0.60-1.45) for NOAC plus DAPT.
If patients under dual or triple therapy need re-angiography, radial access should be the preferred choice in order to reduce risk of periprocedural bleeding. PCI without interruption of VKAs, to avoid bridging therapy that may lead to more bleeding or ischaemic complications, has also been advocated.
CORONARY REVASCULARISATION
Revascularisation for NSTE-ACS relieves symptoms, shortens hospital stay, and improves prognosis. The indications and timing for myocardial revascularisation and choice of preferred approach (PCI or CABG) depend on many factors including the patient’s condition, the presence of risk features, co-morbidities, and extent and severity of the lesions as identified by coronary angiography.
Risk stratification should be performed as early as possible to rapidly identify high-risk individuals and reduce delay to an early invasive approach. However, patients with NSTE-ACS represent a heterogeneous population in terms of risk and prognosis. This extends from low-risk patients who benefit from conservative treatment and a selective invasive approach to patients at high risk of death and cardiovascular events, who should be rapidly referred for angiography and revascularisation. Therefore, risk stratification is critical for selection of the optimal management strategy. Analysis of patient risk profile may be performed by assessment of generally accepted high-risk criteria and/or applying predefined risk scores such as the GRACE risk score.
Invasive versus conservative approach
Many randomised controlled trials (RCTs) and meta-analyses have assessed the effects of a routine invasive versus conservative or selective invasive approach in the short and long term. The benefit of revascularisation is difficult to compare and tends to be underestimated in these trials due to different proportions of patients crossing over from the conservative arm to revascularisation (crossover rates vary from 28% to as high as 58%). In general, the benefit is more pronounced when the difference in revascularization rates between invasive versus conservative arms is high. Furthermore, the selection of patients may have been biased, as some studies included all consecutive patients while others excluded severely unstable patients.
A meta-analysis of seven RCTs comparing routine angiography followed by revascularisation with a selective invasive strategy showed reduced rates of combined death and MI, with a non-significant trend towards fewer deaths and a significant reduction in MI alone, with a routine invasive strategy . There was, however, an early hazard in terms of a significantly higher risk of death and of death and MI during initial hospitalisation for the routine invasive management. However, four of the seven trials included in this meta-analysis were not contemporary due to marginal use of stents and GP IIb/IIIa receptor inhibitors. Another meta-analysis including seven trials with more contemporary adjunctive medication showed a significant risk reduction for all-cause mortality and non-fatal MI for an early invasive versus conservative approach at 2 years without excess of death and non-fatal MI at 1 month . A more recent meta-analysis of eight RCTs showed a significant reduction in death, MI, or rehospitalisation with ACS for the invasive strategy at 1 year . However, this benefit was driven mainly by improved outcomes in biomarker-positive (high-risk) patients. In a sex-specific analysis, a comparable benefit was found in biomarker-positive women compared with biomarker-positive men. Importantly, biomarker-negative women tended to have a higher event rate with an early invasive strategy, suggesting that early invasive procedures should be avoided in low-risk, troponin-negative, female patients. A recent meta-analysis, based on individual patient data from the Fragmin and Fast Revascularization during Instability in Coronary Artery Disease-II (FRISC II), Invasive versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS), and Randomized Intervention Trial of unstable Angina-3 (RITA-3) studies comparing a routine invasive versus selective invasive strategy, revealed a reduction in rates of death and non-fatal MI at 5-year follow-up, with the most pronounced difference in high-risk patients . Age, diabetes, previous MI, ST-segment depression, hypertension, body mass index (<25 kg/m2 or >35 kg/m2), and treatment strategy were found to be independent predictors of death and non-fatal MI during follow-up . There was a 2.0–3.8% absolute reduction in cardiovascular death or MI in the low- and intermediate-risk groups, and an 11.1% absolute risk reduction in the highest-risk patients. These results support a routine invasive strategy, but highlight the role of risk stratification in the management decision process.
The subgroups of patients at high risk that benefit from an early invasive management (diabetic patients, the elderly and patients with renal insufficiency) are discussed in the respective sections.
Timing of angiography and intervention
The optimal timing of angiography and revascularisation in NSTE-ACS has been studied extensively. However, patients at very high risk, i.e., those with refractory angina, severe heart failure, life-threatening ventricular arrhythmias, or haemodynamic instability, were generally not included in RCTs, in order not to withhold potentially life-saving treatment. Such patients may have evolving MI and should be taken to an immediate (<2 h) invasive evaluation, regardless of ECG or biomarker findings.
Previously, there was a debate about whether early angiography followed by revascularisation is associated with an early hazard. A very early invasive strategy (0.5–14 hours), as opposed to a delayed invasive strategy (21–86 hours), was tested in five prospective RCTs, of which only Timing of Intervention in Patients with Acute Coronary Syndromes (TIMACS) had an adequate size (for overview see the ESC revascularization guidelines. In a meta-analysis of four trials – Angioplasty to Blunt the Rise of Troponin in Acute Coronary Syndromes Randomized for an Immediate or Delayed Intervention (ABOARD), Early or Late Intervention in unStable Angina (ELISA), Intracoronary Stenting With Antithrombotic Regimen Cooling Off (ISAR-COOL), and TIMACS – early catheterisation followed by coronary intervention on the first day of hospitalisation was shown to be safe and superior in terms of lower risk of recurrent ischaemia (–41%) and shorter hospital stay (-28%) . With respect to hard endpoints, only the small OPTIMA trial found an increased rate of procedure-related MI in patients having immediate (30 min) compared with a deferred (25 hours) strategy . In contrast, the ABOARD trial did not confirm a difference in MI as defined by troponin release when an immediate intervention (1.2 h) was compared with a strategy of intervention deferred to the next working day (mean 21 h) .
Owing to heterogeneous risk profiles, the optimal timing for an invasive approach may vary in different risk cohorts. There is growing evidence to suggest a benefit of an invasive strategy within 24 hours in patients with a high-risk profile. The TIMACS trial revealed a significant 38% reduction in death, MI, or stroke at 6 months in high-risk patients (GRACE score >140), with an early (≤24 hours) compared to a delayed (≥36 hours) strategy. No significant difference was observed in patients with a low- to intermediate-risk profile (GRACE score ≤140) . Importantly, there were no safety issues regarding an early invasive strategy in this trial. In the ACUITY data analysis, delay to PCI >24 hours was an independent predictor of 30-day and 1-year mortality. This increased ischaemic event rate was most evident among moderate- and high-risk patients (according to the TIMI risk score).
Optimal adjunctive pharmacotherapy is important in an invasive strategy, but pretreatment should not delay angiography and the intervention. An intentional delayed invasive approach for stabilisation including GP IIb/IIIa receptor inhibitors (“cooling-off” strategy) is of no benefit .
In summary, timing of angiography and revascularisation should be based on patient risk profile. Patients at very high risk (as defined above) should be considered for urgent coronary angiography (<2 hours). In patients at high risk with a GRACE risk score of >140 or with at least one major high-risk criterion, an early invasive strategy with catheterization within 24 hours appears to be the most reasonable time window. This implies expedited transfer for patients admitted to hospitals without onsite catheterisation facilities. In lower-risk subsets with a GRACE risk score of <140 but with at least one high-risk criterion (Table 8 and 9), the invasive evaluation can be delayed without increased risk but should be performed during the same hospital stay, preferably within 72 hours of admission. In such patients immediate transfer is not mandatory, but should be organised within 72 hours. In other low-risk patients without recurrent symptoms a non-invasive assessment of inducible ischaemia should be performed before hospital discharge. Coronary angiography should be performed if the results are positive for reversible ischaemia.
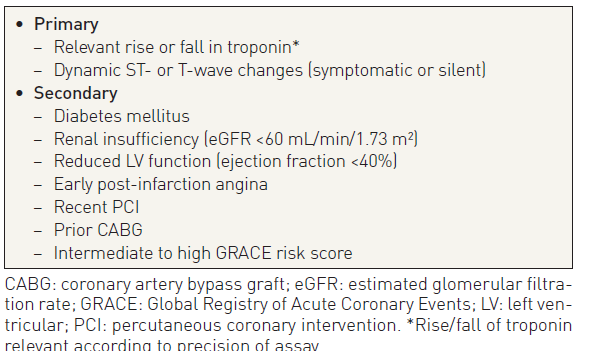
Table 8
Percutaneous coronary intervention versus coronary artery bypass surgery
There are no specific RCTs comparing PCI with CABG in patients with NSTE-ACS. In all trials comparing an early with a late strategy, or an invasive with a medical management strategy, the decision regarding whether to perform CABG or PCI was left to the discretion of the investigator.
In patients stabilised after an episode of ACS, the choice of revascularisation modality can be made as in stable CAD .
In approximately one-third of patients angiography will reveal single-vessel disease, allowing ad hoc PCI in most cases. Multivessel disease will be present in another 50% . Here the decision is more complex and the choice has to be made between culprit lesion PCI, multivessel PCI, CABG, or a combined (hybrid) revascularisation in some cases. The revascularisation strategy should be based on the clinical status as well as the severity and distribution of the CAD and the lesion characteristics.
Culprit lesion PCI usually is the first choice in most patients with multivessel disease. The strategy of multivessel stenting for suitable significant stenoses rather than stenting the culprit lesion only has not been evaluated appropriately in a randomised fashion. However, in a large database including 105, 866 multivessel CAD patients with NSTE-ACS, multivessel PCI was compared with single-vessel PCI . Multivessel PCI was associated with lower procedural success but similar in-hospital mortality and morbidity, although no long-term results were reported.
CABG was compared with PCI in a propensity-matched analysis among patients with multivessel disease from the ACUITY trial . PCI-treated patients had lower rates of stroke, MI, bleeding, and renal injury, similar 1-month and 1-year mortality, but significantly higher rates of unplanned revascularisation at both 1 month and 1 year. However, only 43% of CABG patients could be matched and there was a strong trend for a greater major adverse cardiac event rate at 1 year with PCI compared with CABG (25.0% vs. 19.5%; p= 0.05). These results are consistent with those of the SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) trial, which included 28.5% of patients with a recent ACS, in both PCI and CABG arms . As up to one third of patients with multi-vessel CAD who were included in these trials presented with unstable angina or NSTE-ACS, it is reasonable to use criteria applied in patients with stable CAD to guide the choice of revascularization modality among patients stabilised with NSTE-ACS .
Culprit lesion PCI does not necessarily require a case-by-case review by the “Heart Team” (a multidisciplinary decision-making team), when on clinical or angiographic grounds the procedure needs to be performed ad hoc after angiography . However, protocols based on the SYNTAX score should be designed by the Heart Team at each institution, defining specific anatomical criteria and clinical subsets that can be treated ad hoc or transferred directly to CABG. After culprit lesion PCI, patients with scores in the two higher terciles of the SYNTAX score should be discussed within the Heart Team, in light of functional evaluation of the remaining lesions. This also includes the assessment of co-morbidities and individual characteristics.
Coronary artery bypass surgery
The proportion of patients with NSTE-ACS undergoing bypass surgery during initial hospitalisation is approximately 10%. While the benefit from PCI in patients with NSTE-ACS is related to its early performance, the benefit from CABG is greatest when patients can be operated on after several days of medical stabilisation depending on individual risk. As there is no randomised study comparing an early with a delayed CABG strategy, the general consensus is to wait for 48–72 hours in patients who had culprit lesion PCI and have additional severe CAD. In a large database analysis of unselected patients admitted for ACS, performance of early CABG, even in higher-risk patients, was associated with very low in-hospital mortality . In the CRUSADE and ACTION registry–Get With The Guidelines programmes in patients with NSTEMI, unadjusted and adjusted analyses showed no difference in outcomes between patients undergoing early (≤48 hour) or in-hospital late (>48 hour) surgery, although CABG was delayed more often in higher-risk patients, suggesting that timing might be appropriately determined by multidisciplinary clinical judgement . Therefore in patients selected for CABG, its timing should be individualised according to symptoms, haemodynamic status, coronary anatomy, and inducible ischaemia or flow reserve measurements. When there is ongoing or recurrent ischaemia, ventricular arrhythmias, or haemodynamic instability, CABG should be performed immediately. Surgery should be performed during the same hospital stay in patients with left-main or three-vessel disease involving the proximal left anterior descending artery. In this decision process it is important to consider the risk of bleeding complications in patients who undergo bypass surgery, when initially treated with aggressive antiplatelet treatment. However, pretreatment with a triple or dual antiplatelet regimen should be considered only as a relative contraindication to early bypass surgery, but does require specific surgical measures to minimise bleeding. In patients requiring emergent surgery before the washout period of thienopyridines, off-pump CABG or minimised cardiopulmonary bypass circuits, blood salvaging techniques, and platelet transfusion should be used to minimise risk of bleeding and its consequences.
Percutaneous coronary intervention technique
Outcome after PCI in NSTE-ACS has been improved markedly with the use of intracoronary stenting and contemporary antithrombotic and antiplatelet therapies. As for all patients undergoing PCI, stent implantation in this setting helps to reduce the threat of abrupt closure and restenosis. In line with comparable safety and superior efficacy, new-generation DES are recommended over BMS for patients with NSTE-ACS . In addition, HORIZONS AMI, a randomised study of DES versus BMS in STEMI patients, did not reveal any safety concerns, whereas a consistent reduction in the incidence of restenosis and unplanned repeat revascularisation was found after DES implantation . As mentioned above, DAPT is recommended for 12 months independent of stent type. In patients with a compelling indication for long-term anticoagulation, triple therapy with aspirin, clopidogrel, and OAC should be given for 4 weeks and followed by 12 months of dual therapy with clopidogrel and OAC/NOAC. Dual therapy may be considered as an alternative to triple therapy in patients with high bleeding risk. The use of aspiration thrombectomy in the NSTEMI setting is possible; however, its benefit was not assessed prospectively in randomised trials in patients with NSTE-ACS . It remains undetermined whether other coronary segments with insignificant stenosis but vulnerable features merit mechanical intervention; such intervention is therefore not supported by evidence.
SPECIAL POPULATIONS AND CONDITIONS
The elderly
The clinical presentation of NSTE-ACS in the elderly is often atypical and they are more likely to have mild symptoms. Among elderly patients with atypical presentation of NSTE-ACS, dyspnoea is the leading symptom while syncope, malaise, and confusion are less frequent. The results of an ECG are less likely to demonstrate marked ST-segment deviation. Elderly patients present more frequently with NSTE-ACS than STEMI.
Age is one of the most important predictors of risk in NSTE-ACS . Patients aged >75 years have at least double the mortality rate of those <75 years. The prevalence of ACS-related complications such as heart failure, bleeding, stroke, renal failure, and infections markedly increases with age.
The risk of major bleeding associated with unfractionated heparin, enoxaparin, GP IIb/IIIa receptor inhibitors, and P2Y12 inhibitors is significantly increased in older patients. In the SYNERGY trial, no difference in the rates of 30-day death or MI, 30-day death, and 1-year death between UFH and enoxaparin groups was observed among patients >75 years of age. However, the rates of TIMI major bleeding and GUSTO severe bleeding were significantly higher in the enoxaparin group. As a consequence, enoxaparin should be used with caution in the elderly and the dose should be adapted to renal function. Over 75 years of age, the dose should be reduced to 1 mg/kg once daily and anti-Xa activity monitored . A significantly lower risk of bleeding was observed with fondaparinux compared with enoxaparin in patients >65 years of age in the OASIS-5 trial .
Elderly patients are substantially less likely to undergo an invasive strategy after NSTE-ACS. However, reports from individual trials suggested that the benefit from the invasive strategy was mainly observed in patients >65 years of age. In a subgroup analysis of the Treat angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy (TACTICS)-TIMI 18 trial, patients >75 years of age with NSTE-ACS derived the largest benefit, both in terms of relative and absolute risk reductions, from an invasive strategy at the cost of an increase in risk of major bleeding and need for transfusions. This was confirmed by a detailed meta-analysis .
Decisions on how to manage individual elderly patients should be based on ischaemic and bleeding risk, estimated life expectancy, co-morbidities, quality of life, patient wishes and the estimated risks and benefits of revascularisation.
Gender
Women presenting with NSTE-ACS are older than men and have a higher frequency of diabetes, hypertension, heart failure, and other co-morbidities. Atypical presentations, including dyspnoea or symptoms of heart failure, are more common. Although no sex-specific treatment effect has been described for most therapeutic agents, women with NSTE-ACS are less likely than men to receive evidence-based therapies including invasive diagnostic procedures and coronary revascularisation.
Contradictory results have been published with respect to the influence of sex on the treatment effect of an invasive strategy in NSTE-ACS. While observational studies suggested better long-term outcomes in unselected women undergoing an early invasive strategy, a meta-analysis showed that the benefit of invasive strategies was restricted to male patients, with no benefit in women up to 1 year of follow-up .
The meta-analysis by the Cochrane collaboration pointed out that women derive a significant long-term benefit in terms of death or MI (RR 0.73; 95% CI 0.59–0.91) for an invasive versus conservative strategy, although with an early hazard (Interventions for patients with diabetes mellitus).
Diabetes mellitus
Diabetes mellitus is an independent predictor of mortality among patients with NSTE-ACS. Registries have consistently shown that revascularisation (any form), thienopyridines, and GP IIb/IIIa receptor inhibitors were prescribed less frequently among diabetic patients than among non-diabetic patients, with a clear impact on in-hospital and long-term mortality (5.9% vs. 3.2% at 1 month, and 15.2% vs. 7.6% at 1 year). In addition, diabetic patients are less likely to receive reperfusion therapies or undergo revascularisation compared with non-diabetic patients.
Revascularisation in diabetic patients causes specific problems. CAD is typically diffuse and extensive, and restenosis as well as occlusion rates after PCI and CABG are higher. Repeat revascularisation procedures are more frequent after PCI, compared with CABG. An early invasive approach has been shown to be beneficial in this high-risk subgroup, with greater benefit in diabetic than in non-diabetic patients .
In unselected diabetic patients with multivessel disease, CABG appears to offer a better outcome compared with PCI. In a meta-analysis of individual data from 7,812 patients in 10 randomised trials, CABG was associated with significantly lower mortality at 5.9-year follow-up than with PCI in diabetic patients . Overall there was no difference in mortality with CABG versus PCI (15% vs. 16%; HR 0.91; 95% CI: 0.82–1.02; p= 0.12), but mortality was significantly lower for CABG among 1, 233 patients with diabetes (23% vs. 29%; HR 0.70; 95% 0.56–0.87; p= 0.05; numbers needed to treat [NNT] = 17). In the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI-2D) trial, diabetic patients with stable angina were randomised to either intensive medical therapy or intensive medical therapy plus revascularisation with either CABG or PCI (physician’s choice). At 5-year follow-up, in 763 patients in the CABG group, the rates of all-cause mortality or MI were significantly lower in the CABG group versus intensive medical therapy alone (21.1% vs. 29.2%; p <0.010), as well as the rate of cardiac death or MI (15.8% vs. 21.9%; p <0.03) and MI (10% vs. 17.6%; p <0.003). There was no significant difference in outcome between intensive medical therapy alone and intensive medical therapy plus PCI , . In SYNTAX – a trial comparing CABG to PCI with DES in main stem and multivessel disease – the difference in major adverse cardiac and cerebral events at 1-year follow-up between CABG and PCI groups was doubled in the predefined diabetes-cohort, mostly driven by repeat revascularisation. However, there was no significant difference in rates of death or MI. Finally, in the New York Registry, a trend to improved outcomes in diabetic patients treated with CABG compared with DES (OR for death or MI at 18 months 0.84; 95% CI: 0.69–1.01) was reported .
All of these studies suggest that CABG offers a better outcome compared with PCI in diabetic patients. However, it has to be pointed out that these trials incorporated mostly – if not only – chronic stable patients, and it is unclear whether these data can be extrapolated to patients with NSTE-ACS.
With respect to the choice of stent, in a meta-analysis DES proved to be at least as safe as BMS provided that DAPT is continued for >6 months, which is indicated in ACS anyway . Repeat target vessel revascularisation was considerably less frequent with DES than BMS (OR 0.29 for sirolimus-eluting; 0.38 for paclitaxel-eluting). It may be assumed that this is similar in diabetic patients with ACS. Regarding the choice of conduits, observational studies suggest that arterial grafts offer better outcome compared with saphenous vein grafts. The impact of revascularisation with bilateral arterial grafting on long-term outcome and risk of mediastinal infections is still debated. Again, no data confined to ACS patients alone are available.
There is no indication that the antithrombotic regimen should differ between diabetic and non-diabetic patients. However, in the TRITON-TIMI 38 trial, prasugrel was shown to be superior to clopidogrel in reducing the composite endpoint of cardiovascular death or MI or stroke without excess of major bleeding. Similarly, ticagrelor, when compared with clopidogrel in the PLATO trial, reduced the rate of ischaemic events in ACS patients irrespective of diabetic status and glycaemic control, without an increase in major bleeding events. Ticagrelor reduced all-cause mortality in patients with haemoglobin A1c above the median (>6%). Although GP IIb/IIIa receptor inhibitors were shown in an earlier meta-analysis (without concomitant use of thienopyridines) to have a favourable impact on outcome in diabetic patients, routine upstream treatment was not confirmed to be beneficial in the more recent EARLY-ACS trial . Therefore, with the current use of high-dose oral antiplatelet agents, diabetic patients do not seem to benefit from the routine addition of GP IIb/IIIa receptor inhibitors.
Prevention of contrast-induced nephropathy is particularly important in diabetic patients undergoing angiography and/or PCI. There are no data to support delay of angiography in patients treated with metformin as the risk of lactate acidosis is negligible. Renal function should be monitored closely following contrast exposure (Lesion and patient subsets: chronic kidney disease (old)).
Chronic kidney disease
Renal dysfunction is present in 30%–40% of patients with NSTE-ACS. CKD is an independent predictor of short- and long-term mortality and of major bleeding in patients with NSTE-ACS. Despite the fact that patients with NSTE-ACS and CKD are frequently underrepresented in clinical trials, there is no particular reason not to treat these patients just like patients without renal dysfunction. However, caution is needed with respect to the antithrombotic treatment in terms of bleeding complications See recommendations in Table 10. Registry data show that CKD patients are often overdosed with antithrombotics, particularly anticoagulants and GP IIb/IIIa receptor inhibitors, and are therefore more prone to bleed . In case of severe renal failure, when fondaparinux or enoxaparin are contraindicated, UFH should be used since its activity is easily monitored with aPTT, and it can be quickly neutralised in the event of bleeding.
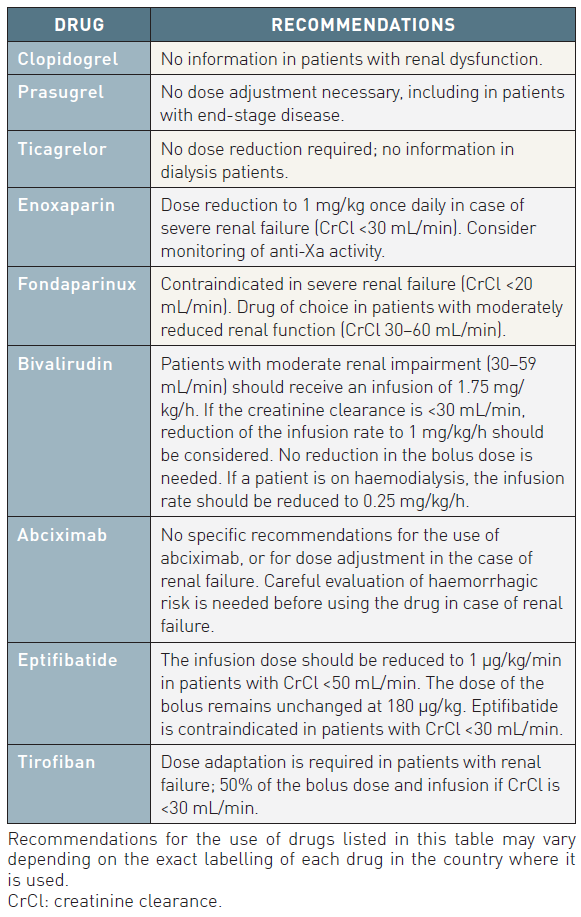
Table 10
Data on the impact of an invasive strategy on clinical endpoints in patients with NSTE-ACS and CKD are not available, as many trials of revascularisation in NSTE-ACS excluded patients with CKD. In a large registry as well as in sub-studies of trials in the setting of NSTE-ACS, outcome of CKD patients improved with invasive management, not only at end-stage renal failure but also at the stage of moderate CKD. In observational studies an early invasive therapy is associated with better 1-year survival in patients with mild-to-moderate renal insufficiency, but the benefit decreases with worse renal function, and is uncertain in those with renal failure or on dialysis.
Patients with CKD are at risk of contrast-induced nephropathy. This risk is increased in patients with older age and diabetes. In case of urgent angiography the risk of contrast-induced nephropathy must be balanced against the ischaemic risk. Hydration before (12 hours) and following (24 hours) angiography and/or angioplasty is the strategy that has been shown to have the greatest impact in reducing the risk of this nephropathy. The amount of contrast should be maintained <4 mL/kg.
Left ventricular systolic dysfunction and heart failure
Heart failure is one of the most frequent and deadly complications of NSTE-ACS. In patients presenting with heart failure without chest pain, ACS may be difficult to diagnose due to a troponin rise related to acute heart failure. In these patients it might be impossible to distinguish acute heart failure alone, from NSTEMI complicated with heart failure. Coronary angiography may be helpful to differentiate the two conditions. Patients with NSTE-ACS and heart failure less frequently receive evidence-based therapies, including beta-blockers and ACE-inhibitors or angiotensin receptor blockers (ARBs), coronary angiography and revascularisation. All recommendations derived from post-MI studies, may be extrapolated to NSTE-ACS patients with heart failure and are found in the respective guidelines .
Extreme body weights
Low body weight is associated with an increased risk of death or MI, and particularly of bleeding, which is frequently due to inappropriate dosing of antithrombotic drugs. Although obesity is associated with a higher risk of coronary events in the population, obese patients with NSTE-ACS show better in-hospital and 1-year outcomes, including lower bleeding risk, which has been called the “obesity paradox” .
Non-obstructive coronary artery disease
A sizeable proportion of patients (about 15%) with NSTE-ACS have normal coronary arteries or non-obstructive lesions (MINOCA). The pathophysiology of NSTE-ACS is not homogeneous and possible mechanisms include: a coronary artery spasm (Prinzmetal’s angina), an intramural plaque complicated by acute thrombosis with subsequent recanalisation, coronary emboli, spontaneous coronary dissection (SCAD) and “syndrome X”.
In patients admitted with suspected NSTE-ACS, the demonstration of normal or near-normal coronary arteries at angiography challenges the diagnosis. However, ST-segment changes and release of biomarkers in patients with typical chest pain and patent coronary arteries without significant stenotic lesions may be due to true necrosis rather than false-positive results. This tends to be more common in women. Relevant atherosclerotic burden may be present even in the absence of angiographically significant stenoses because it may occur in a diffuse manner and lead to arterial wall remodelling in which the wall thickens and expands outwards without encroaching on the lumen. The prognosis of these patients appears to be better than that of patients with NSTE-ACS and significant coronary atherosclerosis, and they therefore merit optimal antithrombotic therapy and secondary prevention with antiplatelet agents and statins.
Prinzmetal’s variant angina refers to a frequently unrecognised syndrome of chest pain secondary to myocardial ischaemia that is not precipitated by physical exertion or emotional stress, and is associated with transient ST-segment elevation. The underlying pathological mechanism is spasm of an epicardial coronary artery that may occur at sites of severe focal stenoses, but typically is seen on angiography at sites of minimal atherosclerotic disease. The spasm may be spontaneous or provoked by an acetylcholine, a “cold pressor” test, or hyperventilation. The mainstay therapy for Prinzmetal’s angina is the administration of calcium antagonists, shown to be effective in preventing coronary spasm, alone or in combination with nitrates. They should be prescribed at maximally tolerated doses.
Another reason might be a spontaneous coronary dissection. The true prevalence of SCAD remains uncertain, primarily because it is an underdiagnosed condition. Missed diagnoses are driven by a low suspicion of ACS in young women even in the presence of classic presenting symptoms, limitations of current coronary angiographic techniques, and lack of clinician familiarity with the condition. SCAD most commonly occurs in patients with few or no cardiovascular risk factors. Recent series using careful diagnostic criteria that exclude iatrogenic, traumatic, and atherosclerotic dissection suggest that SCAD may be a cause of up to 1% to 4% of ACS cases overall , , . It occurs more often in women and may be the cause of ACS in up to 35% of ACS in women ≤50 years of age. It is the most common cause of pregnancy-associated MI (43%) . Intracoronary imaging has become instrumental to aid the diagnosis of SCAD in angiographically subtle or nondiagnostic cases. It can detect intimal tear, false lumen, and intraluminal thrombi, although its resolution may not be adequate to clearly identify all such abnormalities related to SCAD. No comprehensive prospective studies have routinely performed angiographic restudy after SCAD, but observational data have indicated angiographic “healing” of SCAD lesions in the majority of patients (70%–97%) who were selectively restudied weeks to months after a conservatively managed index episode. In current clinical practice, the duration of hospitalization after ACS is therefore largely dictated by the management strategy for the patient and the ongoing symptoms. A prolonged period of in-hospital monitoring (3–5 days) is justified as part of a conservative strategy for patients suffered from ACS caused by SCAD to observe for a small but important (5%–10%) early hazard of dissection extension or new recurrent SCAD. If clinical worsening occurs despite conservative management (worsening of symptoms with evidence of ischemia by electrocardiography or significant arrhythmia), repeat angiography should be performed and emergency revascularization undertaken to relieve ischemia if feasible .
The term “syndrome X” is used to describe patients with angina precipitated by exercise, ST-segment depression on stress test, and non-obstructed coronary arteries at angiography. There is growing evidence that such patients often have an increased response to pain. Because the prognosis is excellent, the most important therapy is reassurance and symptom relief, for which nitrates, beta-blockers, and calcium antagonists have been found to be effective.
Apical ballooning (Tako-Tsubo cardiomyopathy) may present clinically as STEMI or NSTE-ACS and is characterised by normal coronary arteries at angiography accompanied by apical and sometimes medioventricular or basal akinesia unrelated to the distribution of a coronary artery. It is more frequent in women and occurs typically after major emotional stress. The LV dysfunction is generally reversible within days to weeks.
In rare cases, NSTE-ACS with a normal or near-normal coronary arteriogram is linked to coronary embolism, due to atrial fibrillation or atrial flutter. As atrial fibrillation is often clinically unrecognised, the frequency of this mechanism of NSTE-ACS may be underestimated.
Regarding to this additional diagnostic such as MR-scan should always be considered in patients with NSTE-ACS if reason could not be indentified by coronary angiography and intravascular imaging.
Bleeding and transfusion
Bleeding is the most frequent non-ischaemic complication observed in the management of NSTE-ACS, as well as in other clinical settings such as STEMI, PCI and cardiac surgery.
Because of the lack of a universally accepted definition for bleeding, its true frequency is still difficult to assess across trials and registries. Irrespective of the scale used to assess bleeding, many reports confirmed the dose-dependent association between bleeding and risk of death or other ischaemic events. Major bleeding was shown to be associated with a fourfold increase in the risk of death, a fivefold increase in risk of recurrent MI, and a threefold increase in risk of stroke at 30 days. Bleeding risk is highest during the first 30 days, but long-term exposure to potent antiplatelet therapy leads to a persistent increase in the risk of bleeding.
The independent predictors of major bleeding, established from trials and registries, are baseline characteristics, particularly age, female sex, history of bleeding, baseline haemoglobin, diabetes and renal insufficiency. Bleeding risk increases with the number of antithrombotic drugs in use, including anticoagulants, aspirin, P2Y12 receptor inhibitors, and particularly GP IIb/IIIa receptor inhibitors, as well as use of the femoral rather than the radial approach , , .
Management of bleeding complications
Prevention of bleeding has become as important a target as is the prevention of ischaemic events. Therefore, risk assessment in patients with NSTE-ACS needs to address the risk of both thrombotic and bleeding complications. Prevention of bleeding encompasses the choice of safer drugs, appropriate dosage (taking into account age, sex, and CrCl), reduced duration of antithrombotic treatment, use of a combination of antithrombotic and antiplatelet agents according to proven indications, and the choice of a radial over femoral approach if an invasive strategy is used. Use of closure devices and bivalirudin rather than conventional anticoagulants plus GP IIb/IIIa receptor inhibitors was shown to impact favourably on bleeding risk in a pooled analysis of data from the ACUITY and HORIZONS studies .
Antiplatelet and/or anticoagulation agents should not be reintroduced until strict control of the haemorrhage has been obtained for at least 24 hours when stopped due to an acute bleeding event.
Impact of blood transfusion
Blood transfusion has detrimental effects (excess of death and MI, but also lung infections) in many clinical settings, including ACS, PCI, cardiac surgery, and acute critical care . Blood transfusion has a favourable impact if given for haematocrit values <25%, but not above this value . In this regard, a restrictive transfusion policy with a trigger set at 7 g/dL, and a target haemoglobin level of 9–10 g/dL, was shown to derive better clinical outcome than a liberal transfusion policy in the setting of acute care.
Thrombocytopaenia
Thrombocytopaenia can occur during treatment of NSTE-ACS. Thrombocytopaenia is defined as a decrease in platelet count to <100, 000/μL or a drop of >50% from baseline platelet count.
In the ACS setting, there are two main types of drug-induced thrombocytopaenia, i.e., HIT and GP IIb/IIIa receptor inhibitor-induced thrombocytopaenia, with a different prognosis for each type. Heparin-induced thrombocytopaenia must be suspected when there is a drop of >50% in platelet count, or a decrease in platelet count to <100, 000/μL. It occurs in up to 15% of patients treated with UFH, less frequent under LMWH, and is not seen with fondaparinux. Immediate interruption of UFH or LMWH therapy is mandatory as soon as HIT is suspected. Alternative antithrombotic therapy must be introduced, even in the absence of thrombotic complications.
GP IIb/IIIa receptor inhibitor-induced thrombocytopaenia has been reported to occur at rates ranging from 0.5–5.6% in clinical trials, depending on the compound used. Major bleeds are rare, but may be life-threatening. If platelet counts drop below 10, 000/μL, discontinuation of GP IIb/IIIa receptor inhibitors as well as UFH or enoxaparin is recommended. Platelet transfusions are indicated in case of bleeding. Fibrinogen supplementation with fresh frozen plasma or cryoprecipitate either alone or in combination with platelet transfusion has also been advocated.
Management strategy
For every patient, the physician must make an individual decision, taking into account the patient’s history (co-morbid illnesses, age, etc.), his/her clinical condition, findings during the initial assessment on first contact, and the available pharmacological and non-pharmacological treatment options.
Step one: Initial evaluation
Chest pain or discomfort suggestive of ACS or other symptoms will lead to the patient seeking medical attention or hospitalisation. A patient with suspected NSTE-ACS must be evaluated in a hospital and seen immediately by a qualified physician.
The initial step is to assign the patient without delay to a working diagnosis on which the treatment strategy can be based. The assessment criteria are the following:
- Quality of chest pain and a symptom-orientated physical examination;
- Assessment of the likelihood of CAD (e.g., age, risk factors, previous MI, CABG, PCI);
- ECG (to detect ST segment deviation or other abnormality).
On the basis of these findings, which should be available within 10 minutes of first medical contact, the patient can be assigned to one of the three major working diagnoses:
- STEMI;
- NSTE-ACS;
- ACS (highly) unlikely.
On arrival of the patient in hospital blood is drawn and the results should be available within 60 min to be used in the second step. Initial blood tests must at least include: troponin T or I, creatinine, haemoglobin, blood glucose, thyroid stimulating hormone and blood cell count, in addition to standard biochemistry tests.
Step two: Diagnosis validation and risk assessment
After the patient is assigned to the group NSTE-ACS, IV and oral antithrombotic treatments will be started according to Table 11A and 11B. Further management of the patient will be based on additional information/data:
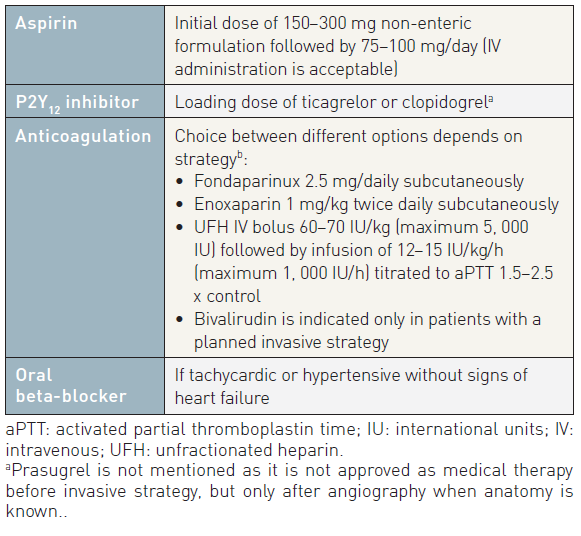
Table 11A
- responsiveness to anti-anginal treatment;
- routine clinical chemistry, particularly troponins (on presentation and after 6–9 hours) and other markers, according to working diagnoses (e.g., D-dimers, BNP, NT-proBNP); if highly sensitive troponins assays are available, a fast track rule-out protocol (3 hours) may be implemented.
- repeat or continuous ST-segment monitoring (when available);
- ischaemic risk-score assessment (GRACE score);
- echocardiogram;
- optional: chest x-ray, CT, MRI or nuclear imaging for differential diagnoses (e.g., aortic dissection, pulmonary embolism, etc.);
- bleeding risk assessment (CRUSADE score).
During step two, other diagnoses may be confirmed or excluded, such as pulmonary embolism and aortic aneurysm.
Risk assessment is an important component of the decision-making process and is subject to constant re-evaluation. It encompasses assessment of both ischaemic and bleeding risk. The risk factors for bleeding and ischaemic events overlap considerably, with the result that patients who are at high risk of ischaemic events are also at high risk of bleeding.
Step three: invasive strategy
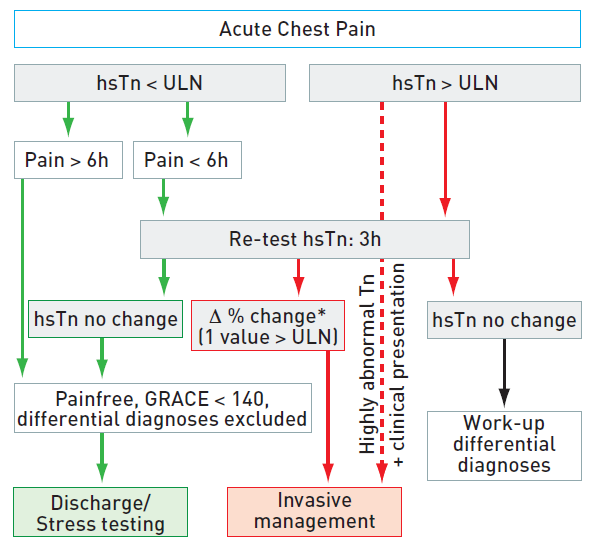
Figure 4a
Rapid rule out of ACS with high sensitive troponin
hsTn: high sensitivity troponin.
ULN: upper limit of normal, 99th percentile of healthy controls.
*Δ% change, dependent on assay.
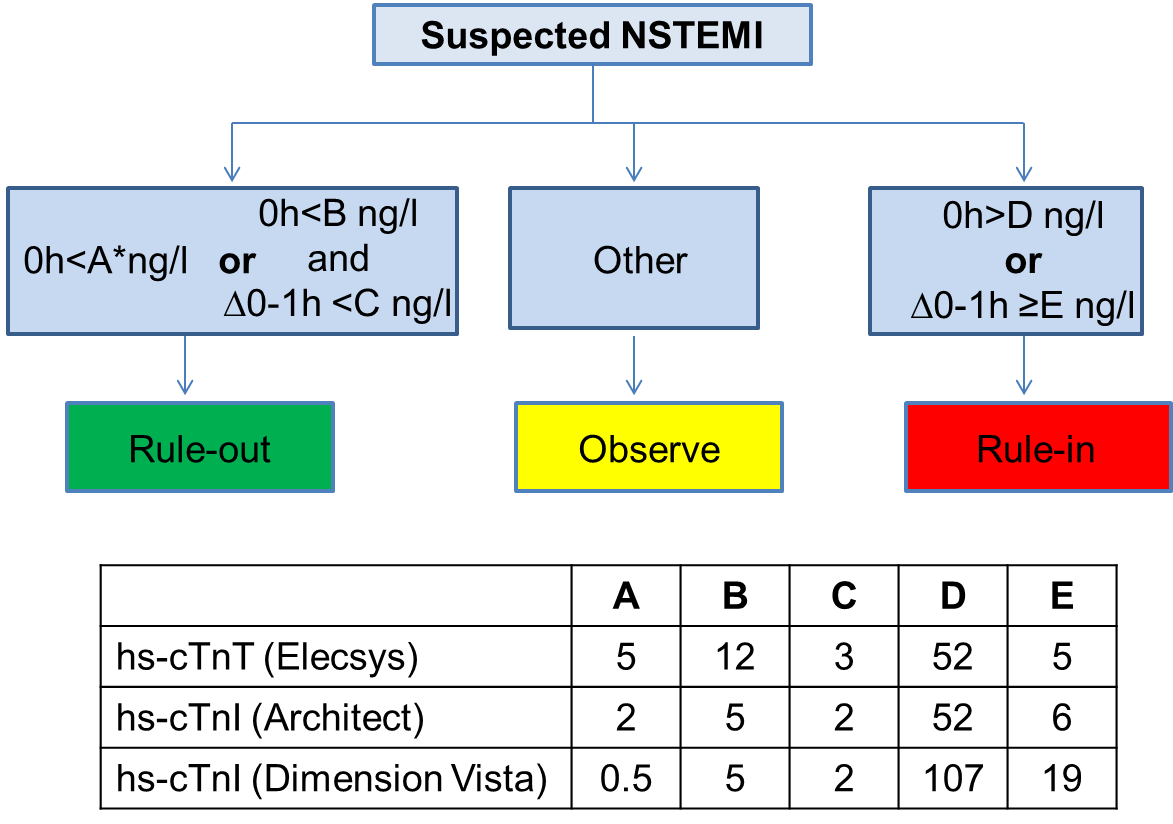
Figure 4b
Suspected NSTEMI
Cardiac catheterisation followed by revascularisation has been shown to prevent recurrent ischaemia and/or improve short-term and long-term outcomes. Several risk factors (troponin elevation, diabetes, ST-depression, renal insufficiency, etc.) have been identified to predict the long-term benefit of an invasive strategy. Depending on the acuteness of risk, the timing of angiography can be tailored, according to four categories (Figure 5):
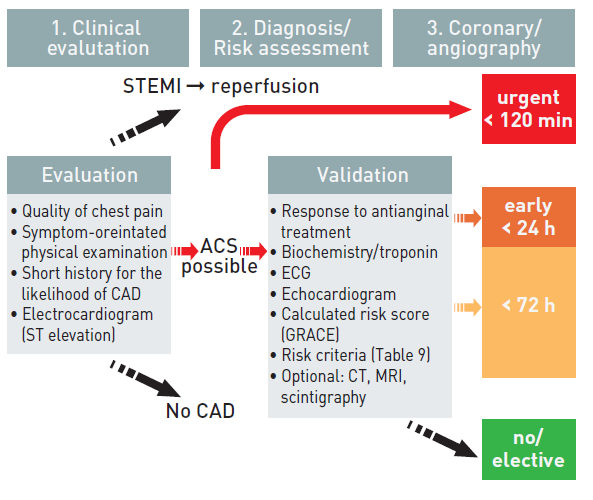
Figure 5
- invasive (<72 hours);
- urgent invasive (<120 min);
- early invasive (<24 hours) - primarily conservative.
The optimal timing depends on the risk profile of the individual patient and can be assessed by several variables.
Urgent invasive strategy (<120 min after first medical contact) should be undertaken for very high-risk patients. These patients are characterised by:
- Refractory angina (indicating evolving MI without ST abnormalities);
- Recurrent angina despite intense anti-anginal treatment, associated with ST depression (2 mm) or deep negative T waves;
- Clinical symptoms of heart failure or haemodynamic instability (“shock”);
- Life-threatening arrhythmias (ventricular fibrillation or ventricular tachycardia).
A GP IIb/IIIa receptor inhibitor (eptifibatide or tirofiban) may be considered in patients with such features in order to bridge the time to catheterisation. A checklist of antithrombotic treatments prior to PCI is given in Table 6.
EARLY INVASIVE STRATEGY (<24 HOURS AFTER FIRST MEDICAL CONTACT)
Most patients initially respond to the anti-anginal treatment, but are at increased risk and need angiography followed by revascularisation. High-risk patients as identified by a GRACE risk score >140 and/or presence of at least one primary high-risk criterion (Table 8) should undergo invasive evaluation within 24 hours.
INVASIVE STRATEGY (<72 HOURS AFTER FIRST MEDICAL CONTACT)
In patients with less-acute risk, according to Table 8, and without recurrence of symptoms, angiography may be performed within a time window of 72 hours. Thus, such patients should undergo elective invasive evaluation at the first opportunity depending on the local circumstances.
Conservative strategy (no or elective angiography)
Patients who fulfil all of the following criteria may be regarded as low risk and should not routinely be submitted to early invasive evaluation:
- No recurrence of chest pain;
- No signs of heart failure;
- No abnormalities in the initial ECG or a second ECG (at 6–9 hours);
- No rise in troponin level (at arrival and at 6–9 hours);
- No inducible ischaemia.
Low risk as assessed by a risk score should support the decision-making process for a conservative strategy. The further management of these patients is according to the evaluation of stable CAD. Before discharge from hospital, a stress test for inducible ischaemia is useful for treatment planning and required before elective angiography.
Step four: revascularisation modalities
If the angiogram shows atheromatous burden but no critical coronary lesions, patients will be referred for medical therapy. The diagnosis of NSTE-ACS may be reconsidered and particular attention paid to other possible reasons for symptoms at presentation, before the patient is discharged. However, the absence of critical coronary lesions does not rule out the diagnosis if the clinical presentation was suggestive of ischaemic chest pain and if biomarkers were positive. In this situation, patients should receive treatment according to the recommendations for NSTE-ACS. Furthermore additional diagnostic steps such as MRI are recommended to identify reasons for elevated biomarkers such as MINOCA.
Recommendations for the choice of a revascularisation modality in NSTE-ACS are similar to those for elective revascularisation procedures. In patients with single-vessel disease, PCI with stenting of the culprit lesion is the first choice. In patients with multivessel disease, the decision for PCI or CABG must be made individually, according to institutional protocols designed by the “Heart Team”. A sequential approach, consisting of treating the culprit lesion with PCI followed by elective CABG with proof of ischaemia and/or functional assessment (FFR) of the non-culprit lesions, may be advantageous in some patients.
The anticoagulant should not be changed during PCI. In patients pre-treated with fondaparinux, UFH must be added before PCI. A GP IIb/IIIa inhibitor should be considered if troponins are elevated or on angiographic presence of thrombus. If CABG is planned, P2Y12 inhibitors should be stopped and surgery deferred only if the clinical condition and the angiographic findings permit.
If angiography shows no options for revascularisation, owing to the extent of the lesions and/or poor distal run-off, freedom from angina at rest should be achieved by intensified medical therapy, and secondary preventive measures should be instituted.
Step five: hospital discharge and post-discharge management
Although in NSTE-ACS most adverse events occur in the early phase, the risk for MI or death remains elevated over several months. Patients treated with early revascularisation are at low (2.5%) risk for developing life-threatening arrhythmias, with 80% occurring during the first 12 hours after onset of symptoms. Accordingly, routine monitoring of the patients beyond 24–48 hours is not warranted. Patients with NSTE-ACS should be hospitalised for at least 24 hours after successful stenting of the culprit lesion.
Intense risk-factor modification and lifestyle change are warranted in all patients following the diagnosis of NSTE-ACS. Enrolment in a cardiac rehabilitation program after discharge can enhance patient adherence to the medical regimen and may be supportive in risk factor modification. A checklist of measures necessary at discharge from hospital is given in Table 12.
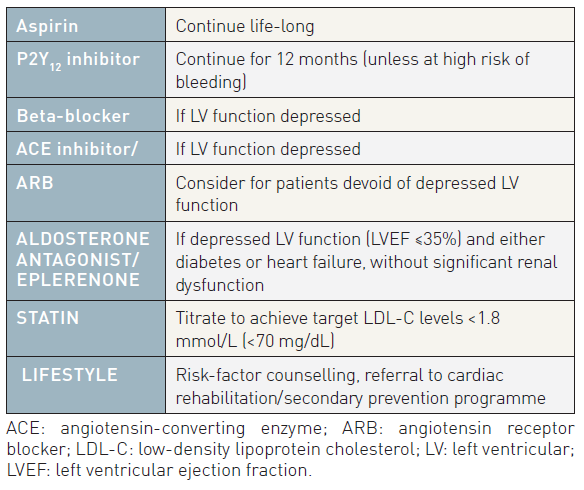
Table 12
Personal perspective - Christian w. Hamm
Coronary interventions in patients with ACS have a prominent role on improving the prognosis and will remain the mainstream treatment. With the current management excellent outcomes can be achieved and there is little room left for major improvements during the acute phase, if guideline recommendations are consequently implemented, like the radial approach and use of prasugrel as antiplatelet agents. However, there is still room to tailor the therapy to individual scenarios especially how the new technical and pharmacological options are best combined to balance the risk of thrombosis and bleeding. Unfortunately, there is limited sponsorship for such trials if there is no incentive for the industry involved. In addition, the focus must be directed to maintain the acute result over long term and to prevent future events by dedicated medical concepts.
- Terkelsen CJ, Lassen JF, Norgaard BL, Gerdes JC, Jensen T, Gotzsche LB, Nielsen TT, Andersen HR. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J. 2005; 26:18-26.
- Universal definition of myocardial infarction. Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Circulation. 2007;116:2634-53.
- Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538-2547.
- Otsuka T, Kawada T, Ibuki C, Seino Y. Association between high-sensitivity cardiac troponin T levels and the predicted cardiovascular risk in middle-aged men without overt cardiovascular disease. Am Heart J. 2010; 159:972-978.
- Aviles RJ, Askari AT, Lindahl B, Wallentin L, Jia G, Ohman EM, Mahaffey KW, Newby LK, Califf RM, Simoons ML, Topol EJ, Berger P, Lauer MS. Troponin T levels in patients with acute coronary syndromes, with or without renal dysfunction. N Engl J Med. 2002; 346:2047-2052.
- Effects of tissue plasminogen activator and a comparison of early invasive and conservative strategies in unstable angina and non-Q-wave myocardial infarction. Results of the TIMI IIIB Trial. Thrombolysis in Myocardial Ischemia. No authors listed. Circulation. 1994;89:1545-56.
- Fragmin and Fast Revascularisation during InStability in Coronary artery disease Investigators. Long-term low-molecular-mass heparin in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet. 1999; 354:701-707.
- Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, Budaj A, Niemela M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011; 377:1409-1420.
- Holmvang L, Clemmensen P, Lindahl B, Lagerqvist B, Venge P, Wagner G, Wallentin L, Grande P. Quantitative analysis of the admission electrocardiogram identifies patients with unstable coronary artery disease who benefit the most from early invasive treatment. J Am Coll Cardiol. 2003; 41905-915.
- Kaul P, Fu Y, Chang WC, Harrington RA, Wagner GS, Goodman SG, Granger CB, Moliterno DJ, Van de Werf F, Califf RM, Topol EJ, Armstrong PW. Prognostic value of ST segment depression in acute coronary syndromes: insights from PARAGON-A applied to GUSTO-IIb. PARAGON-A and GUSTO IIb Investigators. Platelet IIb/IIIa Antagonism for the Reduction of Acute Global Organization Network. J Am Coll Cardiol. 2001; 38:64-71.
- Kaul P, Fu Y, Chang WC, Harrington RA, Wagner GS, Goodman SG, Granger CB, Moliterno DJ, Van de Werf, Califf RM, Topol EJ, Armstrong PW. Prognostic value of ST segment depression in acute coronary syndromes: insights from PARAGON-A applied to GUSTO-IIb. PARAGON-A and GUSTO IIb Investigators. Platelet IIb/IIIa Antagonism for the Reduction of Acute Global Organization Network. J Am Coll Cardiol. 2001; 38:64-71.
- Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000; 284:835-842.
- Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV, Jr., Peterson ED, Alexander KP. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009; 119:1873-1882.
- Theroux P, Ouimet H, McCans J, Latour JG, Joly P, Levy G, Pelletier E, Juneau M, Stasiak J, de Guise P, Pelletier G, Rinzler D, Waters D. Aspirin, heparin, or both to treat acute unstable angina. N Engl J Med. 1988; 319:1105-1111.
- Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, Faxon DP, Rupprecht HJ, Budaj A, Avezum A, Widimsky P, Steg PG, Bassand JP, Montalescot G, Macaya C, Di Pasquale G, Niemela K, Ajani AE, White HD, Chrolavicius S, Gao P, Fox KA, Yusuf S. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010; 376:1233-1243.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001; 345:494-502.
- Montalescot G, Sideris G, Meuleman C, Bal-dit-Sollier C, Lellouche N, Steg PG, Slama M, Milleron O, Collet J-P, Henry P, Beygui F, Drouet L. A Randomized Comparison of High Clopidogrel Loading Doses in Patients With Non-ST-Segment Elevation Acute Coronary Syndromes: The ALBION (Assessment of the Best Loading Dose of Clopidogrel to Blunt Platelet Activation, Inflammation and Ongoing Necrosis) Trial. J Am Coll Cardiol. 2006; 48:931-938.
- Mega JL, Close SL, Wiviott SD, Shen L, Walker JR, Simon T, Antman EM, Braunwald E, Sabatine MS. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010; 376:1312-1319.
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-15.
- Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, Kereiakes DJ, Paris C, Purdy D, Wilson V, Ledley GS, Storey RF. Randomized Double-Blind Assessment of the ONSET and OFFSet of the Antiplatelet Effects of Ticagrelor versus Clopidogrel in Patients with Stable Coronary Artery Disease: The ONSET/OFFSET Study. Circulation. 2009; 120:2577-2585.
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA; PLATO Investigators, Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-57.
- Roffi M, Chew DP, Mukherjee D, Bhatt DL, White JA, Moliterno DJ, Heeschen C, Hamm CW, Robbins MA, Kleiman NS, Theroux P, White HD, Topol EJ. Platelet glycoprotein IIb/IIIa inhibition in acute coronary syndromes. Gradient of benefit related to the revascularisation strategy. Eur Heart J. 2002; 23:1441-1448.
- Stone G, Ware J, Bertrand M, Lincoff A, Moses J, Ohman E, White H, Feit F, Colombo A, McLaurin B, Cox D, Manoukian S, Fahy M, Clayton T, Mehran R, Pocock S. For the ACUITY Investigators. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trial. JAMA. 2007; 298:2497-2506.
- Giugliano RP, White JA, Bode C, Armstrong PW, Montalescot G, Lewis BS, van ‘t Hof A, Berdan LG, Lee KL, Strony JT, Hildemann S, Veltri E, Van de Werf F, Braunwald E, Harrington RA, Califf RM, Newby LK; EARLY ACS Investigators. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med. 2009 ;360:2176-90. Epub 2009.
- Kastrati A, Mehilli J, Neumann F-J, Dotzer F, ten Berg J, Bollwein H, Graf I, Ibrahim M, Pache J, Seyfarth M, Schuhlen H, Dirschinger J, Berger PB, Schomig A, for the Intracoronary S, Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 2 Trial I. Abciximab in Patients With Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention After Clopidogrel Pretreatment: The ISAR-REACT 2 Randomized Trial. JAMA. 2006; 295:1531-1538.
- Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008; 29:2909-2945.
- Topol EJ, Moliterno DJ, Herrmann HC, Powers ER, Grines CL, Cohen DJ, Cohen EA, Bertrand M, Neumann F-J, Stone GW, DiBattiste PM, Yakubov SJ, DeLucca PT, Demopoulos L, the TARGET Investigators. Comparison of Two Platelet Glycoprotein IIb/IIIa Inhibitors, Tirofiban and Abciximab, for the Prevention of Ischemic Events with Percutaneous Coronary Revascularization. N Engl J Med. 2001; 344:1888-1894.
- De Luca G, Ucci G, Cassetti E, Marino P. Benefits From Small Molecule Administration as Compared With Abciximab Among Patients With ST-Segment Elevation Myocardial Infarction Treated With Primary Angioplasty: A Meta-Analysis. J Am Coll Cardiol. 2009; 53:1668-1673.
- Jolly SS, Faxon DP, Fox KA, Afzal R, Boden WE, Widimsky P, Steg PG, Valentin V, Budaj A, Granger CB, Joyner CD, Chrolavicius S, Yusuf S, Mehta SR. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes treated with glycoprotein IIb/IIIa inhibitors or thienopyridines: results from the OASIS 5 (Fifth Organization to Assess Strategies in Ischemic Syndromes) trial. J Am Coll Cardiol. 2009;54:468-76.
- White HD, Ohman EM, Lincoff AM, Bertrand ME, Colombo A, McLaurin BT, Cox DA, Pocock SJ, Ware JA, Manoukian SV, Lansky AJ, Mehran R, Moses JW, Stone GW. Safety and Efficacy of Bivalirudin With and Without Glycoprotein IIb/IIIa Inhibitors in Patients With Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention: 1-Year Results From the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) Trial. J Am Coll Cardiol. 2008; 52:807-814.
- Lincoff AM, Steinhubl SR, Manoukian SV, Chew D, Pollack Jr CV, Feit F, Ware JH, Bertrand ME, Ohman EM, Desmet W, Cox DA, Mehran R, Stone GW. Influence of Timing of Clopidogrel Treatment on the Efficacy and Safety of Bivalirudin in Patients With Non-ST-Segment Elevation Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention: An Analysis of the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) Trial. JACC: Cardiovascular Interventions. 2008; 1:639-648.
- Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L, Joyner C, Fox KA. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006; 354:1464-1476.
- Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L, Joyner C, Fox KA. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA. 2006; 2953:1519-1530.
- Eikelboom JW, Anand SS, Malmberg K, Weitz JI, Ginsberg JS, Yusuf S. Unfractionated heparin and low-molecular-weight heparin in acute coronary syndrome without ST elevation: a meta-analysis. Lancet. 2000; 355:1936-1942.
- Cohen M, Theroux P, Borzak S, Frey MJ, White HD, Van Mieghem W, Senatore F, Lis J, Mukherjee R, Harris K, Bigonzi F. Randomized double-blind safety study of enoxaparin versus unfractionated heparin in patients with non-ST-segment elevation acute coronary syndromes treated with tirofiban and aspirin: the ACUTE II study. The Antithrombotic Combination Using Tirofiban and Enoxaparin. Am Heart J. 2002; 144:470-477.
- Ferguson JJ, Califf RM, Antman EM, Cohen M, Grines CL, Goodman S, Kereiakes DJ, Langer A, Mahaffey KW, Nessel CC, Armstrong PW, Avezum A, Aylward P, Becker RC, Biasucci L, Borzak S, Col J, Frey MJ, Fry E, Gulba DC, Guneri S, Gurfinkel E, Harrington R, Hochman JS, Kleiman NS, Leon MB, Lopez-Sendon JL, Pepine CJ, Ruzyllo W, Steinhubl SR, Teirstein PS, Toro-Figueroa L, White H. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004; 292:45-54.
- Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006; 355:2203-2216.
- Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, Bhatt DL, Goodman S, Verheugt FW, Flather M, Huber K, Liaw D, Husted SE, Lopez-Sendon J, De Caterina R, Jansky P, Darius H, Vinereanu D, Cornel JH, Cools F, Atar D, Leiva-Pons JL, Keltai M, Ogawa H, Pais P, Parkhomenko A, Ruzyllo W, Diaz R, White H, Ruda M, Geraldes M, Lawrence J, Harrington RA, Wallentin L. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011; 365:699-708.
- Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM. Rivaroxaban in Patients with a Recent Acute Coronary Syndrome. N Engl J Med. 2012;366:9-19.
- Mehta SR, Cannon CP, Fox KA, Wallentin L, Boden WE, Spacek R, Widimsky P, McCullough PA, Hunt D, Braunwald E, Yusuf S. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA. 2005; 293:2908-2917.
- Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol. 2006; 48:1319-1325.
- O’Donoghue M, Boden WE, Braunwald E, Cannon CP, Clayton TC, de Winter RJ, Fox KA, Lagerqvist B, McCullough PA, Murphy SA, Spacek R, Swahn E, Wallentin L, Windhausen F, Sabatine MS. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction: a meta-analysis. JAMA. 2008; 300:71-80.
- Fox KA, Clayton TC, Damman P, Pocock SJ, de Winter RJ, Tijssen JG, Lagerqvist B, Wallentin L; FIR Collaboration. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol. 2010;55(22):2435-45.
- Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez- Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501-55.
- Katritsis DG, Siontis GC, Kastrati A, van’t Hof AW, Neumann FJ, Siontis KC, Ioannidis JP. Optimal timing of coronary angiography and potential intervention in non-ST-elevation acute coronary syndromes. Eur Heart J. 2010; 32:32-40.
- Riezebos RK, Ronner E, Ter Bals E, Slagboom T, Smits PC, ten Berg JM, Kiemeneij F, Amoroso G, Patterson MS, Suttorp MJ, Tijssen JG, Laarman GJ. Immediate versus deferred coronary angioplasty in non-ST-segment elevation acute coronary syndromes. Heart. 2009; 95:807-812.
- Montalescot G, Cayla G, Collet JP, Elhadad S, Beygui F, Le Breton H, Choussat R, Leclercq F, Silvain J, Duclos F, Aout M, Dubois-Rande JL, Barthelemy O, Ducrocq G, Bellemain-Appaix A, Payot L, Steg PG, Henry P, Spaulding C, Vicaut E. Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial. JAMA. 2009; 302:947-954.
- Mehta SR, Granger CB, Boden WE, Steg PG, Bassand JP, Faxon DP, Afzal R, Chrolavicius S, Jolly SS, Widimsky P, Avezum A, Rupprecht HJ, Zhu J, Col J, Natarajan MK, Horsman C, Fox KA, Yusuf S. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009; 360:2165-2175.
- Brener SJ, Milford-Beland S, Roe MT, Bhatt DL, Weintraub WS, Brindis RG. Culprit-only or multivessel revascularization in patients with acute coronary syndromes: an American College of Cardiology National Cardiovascular Database Registry report. Am Heart J. 2008; 155:140-146.
- Ben-Gal Y, Moses JW, Mehran R, Lansky AJ, Weisz G, Nikolsky E, Argenziano M, Williams MR, Colombo A, Aylward PE, Stone GW. Surgical versus percutaneous revascularization for multivessel disease in patients with acute coronary syndromes: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. JACC Cardiovasc Interv. 2010; 3:1059-1067.
- Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, Van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW; SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961-72.
- Monteiro P. Impact of early coronary artery bypass graft in an unselected acute coronary syndrome patient population. Circulation. 2006; 114(1 Suppl):I467-472.
- Parikh SV, de Lemos JA, Jessen ME, Brilakis ES, Ohman EM, Chen AY, Wang TY, Peterson ED, Roe MT, Holper EM. Timing of in-hospital coronary artery bypass graft surgery for non-ST-segment elevation myocardial infarction patients results from the National Cardiovascular Data Registry ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With The Guidelines). JACC Cardiovasc Interv. 2010; 3:419-427.
- Stone GW, Lansky AJ, Pocock SJ, Gersh BJ, Dangas G, Wong SC, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Mockel M, Ochala A, Kellock A, Parise H, Mehran R. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009; 360:1946-1959.
- Lopes RD, Alexander KP, Marcucci G, White HD, Spinler S, Col J, Aylward PE, Califf RM, Mahaffey KW. Outcomes in elderly patients with acute coronary syndromes randomized to enoxaparin vs. unfractionated heparin: results from the SYNERGY trial. Eur Heart J. 2008; 29:1827-1833.
- Bavry AA, Kumbhani DJ, Quiroz R, Ramchandani SR, Kenchaiah S, Antman EM. Invasive therapy along with glycoprotein IIb/IIIa inhibitors and intracoronary stents improves survival in non-ST-segment elevation acute coronary syndromes: a meta-analysis and review of the literature. Am J Cardiol. 2004; 93:830-835.
- Hoenig MR, Doust JA, Aroney CN, Scott IA. Early invasive versus conservative strategies for unstable angina & non-ST-elevation myocardial infarction in the stent era. Cochrane Database Syst Rev. 2006 Jul 19.
- Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, Gibson CM, Braunwald E. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001; 344:1879-1887.
- Hlatky MA, Boothroyd DB, Bravata DM, Boersma E, Booth J, Brooks MM, Carrie D, Clayton TC, Danchin N, Flather M, Hamm CW, Hueb WA, Kahler J, Kelsey SF, King SB, Kosinski AS, Lopes N, McDonald KM, Rodriguez A, Serruys P, Sigwart U, Stables RH, Owens DK, Pocock SJ. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009; 373:1190-1197.
- Chaitman BR, Hardison RM, Adler D, Gebhart S, Grogan M, Ocampo S, Sopko G, Ramires JA, Schneider D, Frye RL. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation. 2009; 120:2529-2540.
- Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009; 360:2503-2515.
- Hannan EL, Wu C, Walford G, Culliford AT, Gold JP, Smith CR, Higgins RS, Carlson RE, Jones RH. Drug-eluting stents vs. coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med. 2008; 358:331-341.
- Stettler C, Allemann S, Wandel S, Kastrati A, Morice MC, Schömig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabaté M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, De Carlo M, Erglis A, Chechi T, Ortolani P, Schalij MJ, Diem P, Meier B, Windecker S, Jüni P. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis.Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. BMJ. 2008;337:1331.
- Collet JP, Montalescot G, Agnelli G, Van de Werf F, Gurfinkel EP, Lopez-Sendon J, Laufenberg CV, Klutman M, Gowda N, Gulba D. Non-ST-segment elevation acute coronary syndrome in patients with renal dysfunction: benefit of low-molecular-weight heparin alone or with glycoprotein IIb/IIIa inhibitors on outcomes. The Global Registry of Acute Coronary Events. Eur Heart J. 2005; 26:2285-2293.
- Dickstein K, Vardas PE, Auricchio A, Daubert JC, Linde C, McMurray J, Ponikowski P, Priori SG, Sutton R, van Veldhuisen DJ, Vahanian A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas P, Widimsky P, Anker SD, Blanc JJ, Gasparini M, Hoes AW, Israel CW, Kalarus Z, Merkely B, Swedberg K, Camm AJ. 2010 Focused Update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur Heart J. 2010; 31:2677-2687.
- Diercks DB, Roe MT, Mulgund J, Pollack CV, Jr., Kirk JD, Gibler WB, Ohman EM, Smith SC, Jr., Boden WE, Peterson ED. The obesity paradox in non-ST-segment elevation acute coronary syndromes: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative. Am Heart J. 2006; 152:140-148.
- Chase AJ, Fretz EB, Warburton WP, Klinke WP, Carere RG, Pi D, Berry B, Hilton JD. Association of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A.L study (Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg). Heart. 2008; 94:1019-1025.
- Agostoni P, Biondi-Zoccai GG, de Benedictis ML, Rigattieri S, Turri M, Anselmi M, Vassanelli C, Zardini P, Louvard Y, Hamon M. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004; 44:349-356.
- Marso SP, Amin AP, House JA, Kennedy KF, Spertus JA, Rao SV, Cohen DJ, Messenger JC, Rumsfeld JS. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010; 303:2156-2164.
- Alexander KP, Chen AY, Wang TY, Rao SV, Newby LK, LaPointe NM, Ohman EM, Roe MT, Boden WE, Harrington RA, Peterson ED. Transfusion practice and outcomes in non-ST-segment elevation acute coronary syndromes. Am Heart J. 2008; 155:1047-1053.
- Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, Daly C, De Backer G, Hjemdahl P, Lopez-Sendon J, Marco J, Morais J, Pepper J, Sechtem U, Simoons M, Thygesen K, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Osterspey A, Tamargo J, Zamorano JL. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006; 27:1341-1381.
- Mueller C. Biomarkers and acute coronary syndromes: an update. Eur Heart J 2014;35:552-556.
- Raskovalova T, Twerenbold R, Collinson PO, Keller T, Bouvaist H, Folli C, Giavarina D, Lotze U, Eggers KM, Dupuy AM, Chenevier-Gobeaux C, Meune C, Maisel A, Mueller C, Labarere J. Diagnostic accuracy of combined cardiac troponin and copeptin assessment for early rule-out of myocardial infarction: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2014;3:18–27.
- Lipinski MJ, Escarcega RO, D’Ascenzo F, Magalhaes MA, Baker NC, Torguson R, Chen F, Epstein SE, Miro O, Llorens P, Giannitsis E, Lotze U, Lefebvre S, Sebbane M, Cristol JP, Chenevier-Gobeaux C, Meune C, Eggers KM, Charpentier S, Twerenbold R, Mueller C, Biondi-Zoccai G, Waksman R. A systematic review and collaborative meta-analysis to determine the incremental value of copeptin for rapid rule-out of acute myocardial infarction. Am J Cardiol. 2014;113:1581–1591.
- Mockel M, Searle J, Hamm C, Slagman A, Blankenberg S, Huber K, Katus H, Liebetrau C, Muller C, Muller R, Peitsmeyer P, von Recum J, Tajsic M, Vollert JO, Giannitsis E. Early discharge using single cardiac troponin and copeptin testing in patients with suspected acute coronary syndrome (ACS): a randomized, controlled clinical process study. Eur Heart J 2015;36:369–376.
- Bandstein N, Ljung R, Johansson M, Holzmann MJ. Undetectable high-sensitivity cardiac troponin T level in the emergency department and risk of myocardial infarction. J Am Coll Cardiol. 2014;63:2569–2578.
- Zhelev Z, Hyde C, Youngman E, Rogers M, Fleming S, Slade T, Coelho H, Jones-Hughes T, Nikolaou V. Diagnostic accuracy of single baseline measurement of Elecsys troponin T high-sensitive assay for diagnosis of acute myocardial infarction in emergency department: systematic review and meta-analysis. BMJ 2015;350:h15.
- Reichlin T, Twerenbold R, Wildi K, Gimenez MR, Bergsma N, Haaf P, Druey S, Puelacher C, Moehring B, Freese M, Stelzig C, Krivoshei L, Hillinger P, Jager C, Herrmann T, Kreutzinger P, Radosavac M, Weidmann ZM, Pershyna K, Honegger U, Wagener M, Vuillomenet T, Campodarve I, Bingisser R, Miro O, Rentsch K, Bassetti S, Osswald S, Mueller C. Prospective validation of a 1-hour algorithm to rule-out and rule-in acute myocardial infarction using a highsensitivity cardiac troponin T assay. CMAJ 2015;187:E243–E252.
- Rubini Gimenez M, Twerenbold R, Jaeger C, Schindler C, Puelacher C, Wildi K, Reichlin T, Haaf P, Merk S, Honegger U, Wagener M, Druey S, Schumacher C, Krivoshei L, Hillinger P, Herrmann T, Campodarve I, Rentsch K, Bassetti S, Osswald S, Mueller C. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am J Med 2015;128:861–870.
- Twerenbold R, Wildi K, Jaeger C, Gimenez MR, Reiter M, Reichlin T, Walukiewicz A, Gugala M, Krivoshei L, Marti N, Moreno Weidmann Z, Hillinger P, Puelacher C, Rentsch K, Honegger U, Schumacher C, Zurbriggen F, Freese M, Stelzig C, Campodarve I, Bassetti S, Osswald S, Mueller C. Optimal cutoff levels of more sensitive cardiac troponin assays for the early diagnosis of myocardial infarction in patients with renal dysfunction. Circulation. 2015;131:2041–2050.
- De Servi S, Goedicke J, Schirmer A, Widimsky P. Clinical outcomes for prasugrel versus clopidogrel in patients with unstable angina or non-ST-elevation myocardial infarction: an analysis from the TRITON-TIMI 38 trial. Eur Heart J Acute Cardiovasc Care. 2014;3:363–372.
- Lindholm D, Varenhorst C, Cannon CP, Harrington RA, Himmelmann A, Maya J, Husted S, Steg PG, Cornel JH, Storey RF, Stevens SR,Wallentin L, James SK. Ticagrelor vs. clopidogrel in patients with non-ST-elevation acute coronary syndrome with or without revascularization: results from the PLATO trial. Eur Heart J. 2014;35:2083–2093.
- Szummer K, Oldgren J, Lindhagen L, Carrero JJ, Evans M, Spaak J, Edfors R, Jacobson SH, Andell P, Wallentin L, Jernberg T. Association between the use of fondaparinux vs low-molecular-weight heparin and clinical outcomes in patients with non-ST-segment elevation myocardial infarction. JAMA. 2015;313:707–716.
- Lip GY, Windecker S, Huber K, Kirchhof P, Marin F, ten Berg JM, Haeusler KG, Boriani G, Capodanno D, Gilard M, Zeymer U, Lane D, Storey RF, Bueno H, Collet JP, Fauchier L, Halvorsen S, Lettino M, Morais J, Mueller C, Potpara TS, Rasmussen LH, Rubboli A, Tamargo J, Valgimigli M, Zamorano JL. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology working group on thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J. 2014;35:3155–3179.
- Valgimigli M, Patialiakas A, Thury A, McFadden E, Colangelo S, Campo G, Tebaldi M, Ungi I, Tondi S, Roffi M, Menozzi A, de Cesare N, Garbo R, Meliga E, Testa L, Gabriel HM, Airoldi F, Ferlini M, Liistro F, Dellavalle A, Vranckx P, BriguoriC; ZEUS Investigators. Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J Am Coll Cardiol. 2015;65:805–815.
- Fiedler KA, Maeng M, Mehilli J, Schulz-Schu¨pke S, Byrne RA, Sibbing D, Hoppmann P, Schneider S, Fusaro M, Ott I, Kristensen SD, Ibrahim T, Massberg S, Schunkert H, Laugwitz K-L, Kastrati A, Sarafoff N. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation:the ISAR-TRIPLE trial. J Am Coll Cardiol. 2015;65:1619–1629.
- Valgimigli M, Tebaldi M, Borghesi M, Vranckx P, Campo G, Tumscitz C, Cangiano E, Minarelli M, Scalone A, Cavazza C, Marchesini J, Parrinello G. Twoyear outcomes after first- or second-generation drug-eluting or bare-metal stent implantation in all-comer patients undergoing percutaneous coronary intervention: a pre-specified analysis from the PRODIGY study (prolonging dual antiplatelet treatment after grading stent-induced intimal hyperplasia study). JACC Cardiovasc Interv. 2014;7:20–28.
- Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2014;35:2541–2619.
- Lagerqvist B, Frobert O, Olivecrona GK, Gudnason T, Maeng M, Alstrom P, Andersson J, Calais F, Carlsson J, Collste O, Gotberg M, Hardhammar P, Ioanes D, Kallryd A, Linder R, Lundin A, Odenstedt J, Omerovic E, Puskar V, Todt T, Zelleroth E, Ostlund O, James SK. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371:1111–1120.
- Valgimigli M, Gagnor A, Calabró P, Frigoli E, Leonardi S, Zaro T, Rubartelli P, Briguori C, Andò G, Repetto A, Limbruno U, Cortese B, Sganzerla P, Lupi A, Galli M, Colangelo S, Ierna S, Ausiello A, Presbitero P, Sardella G, Varbella F, Esposito G, Santarelli A, Tresoldi S, Nazzaro M, Zingarelli A, de Cesare N, Rigattieri S, Tosi P, Palmieri C, Brugaletta S, Rao SV, Heg D, Rothenbühler M, Vranckx P, Jüni P; MATRIX Investigators. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015 Jun 20;385(9986):2465-76.
- Ando G, Capodanno D. Radial Versus Femoral Access in Invasively Managed Patients With Acute Coronary Syndrome: A Systematic Review and Meta-analysis. Annals of internal medicine. 2015;163:932-940.
- Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR, Jr., Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. The New England journal of medicine. 2014;371:2155-2166.
- Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS. Long-term use of ticagrelor in patients with prior myocardial infarction. The New England journal of medicine. 2015;372:1791-1800.
- Udell JA, Bonaca MP, Collet JP, Lincoff AM, Kereiakes DJ, Costa F, Lee CW, Mauri L, Valgimigli M, Park SJ, Montalescot G, Sabatine MS, Braunwald E, Bhatt DL. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J. 2016 Jan 21;37(4):390-9.
- Valgimigli M, Frigoli E, Leonardi S, Rothenbuhler M, Gagnor A, Calabro P, Garducci S, Rubartelli P, Briguori C, Ando G, Repetto A, Limbruno U, Garbo R, Sganzerla P, Russo F, Lupi A, Cortese B, Ausiello A, Ierna S, Esposito G, Presbitero P, Santarelli A, Sardella G, Varbella F, Tresoldi S, de Cesare N, Rigattieri S, Zingarelli A, Tosi P, van ’t Hof A, Boccuzzi G, Omerovic E, Sabate M, Heg D, Juni P, Vranckx P. Bivalirudin or Unfractionated Heparin in Acute Coronary Syndromes. The New England journal of medicine. 2015;373:997-1009.
- Schüpke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wöhrle J, Richardt G, Liebetrau C, Witzenbichler B, Antoniucci D, Akin I, Bott-Flügel L, Fischer M, Landmesser U, Katus HA, Sibbing D, Seyfarth M, Janisch M, Boncompagni D, Hilz R, Rottbauer W, Okrojek R, Möllmann H, Hochholzer W, Migliorini A, Cassese S, Mollo P, Xhepa E, Kufner S, Strehle A, Leggewie S, Allali A, Ndrepepa G, Schühlen H, Angiolillo DJ, Hamm CW, Hapfelmeier A, Tölg R, Trenk D, Schunkert H, Laugwitz KL, Kastrati A; ISAR-REACT 5 Trial Investigators. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2019 Oct 17;381(16):1524-1534.
- Sibbing D1, Aradi D2, Jacobshagen C3, Gross L4, Trenk D5, Geisler T6, Orban M4, Hadamitzky M7, Merkely B8, Kiss RG9, Komócsi A10, Dézsi CA11, Holdt L12, Felix SB13, Parma R14, Klopotowski M15, Schwinger RHG16, Rieber J17, Huber K18, Neumann FJ5, Koltowski L19, Mehilli J20, Huczek Z19, Massberg S20; TROPICAL-ACS Investigators. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. 2017 Oct 14;390(10104):1747-1757.
- Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, Džavík V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta SR, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han YL, Pocock S, Gibson CM. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med. 2019 Nov 21;381(21):2032-2042.
- Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, Hata Y, Yagi M, Suematsu N, Yokomatsu T, Takamisawa I, Doi M, Noda T, Okayama H, Seino Y, Tada T, Sakamoto H, Hibi K, Abe M, Kawai K, Nakao K, Ando K, Tanabe K, Ikari Y, Hanaoka KI, Morino Y, Kozuma K, Kadota K, Furukawa Y, Nakagawa Y, Kimura T; STOPDAPT-2 Investigators. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA. 2019 Jun 25;321(24):2414-2427.
- Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van ’t Hof AW, ten Berg JM; WOEST study investigators.Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013 Mar 30;381(9872):1107-15.
- Lopes RD, Hong H, Harskamp RE, Bhatt DL, Mehran R, Cannon CP, Granger CB, Verheugt FWA, Li J, Ten Berg JM, Sarafoff N, Gibson CM, Alexander JH. Safety and Efficacy of Antithrombotic Strategies in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Network Meta-analysis of Randomized Controlled Trials. JAMA Cardiol. 2019.
- Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, Nordaby M, Kleine E, Harper R, Manassie J, Januzzi JL, Ten Berg JM, Steg PG, Hohnloser SH; RE-DUAL PCI Steering Committee and Investigators. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N Engl J Med. 2017 Oct 19;377(16):1513-1524.
- Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, Averkov O, Bahit MC, Berwanger O, Budaj A, Hijazi Z, Parkhomenko A, Sinnaeve P, Storey RF, Thiele H, Vinereanu D, Granger CB, Alexander JH; AUGUSTUS Investigators. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med. 2019 Apr 18;380(16):1509-1524.
- Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED, Fox KA. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med. 2016 Dec 22;375(25):2423-2434.
- Mortensen KH, Thuesen L, Kristensen IB, Christiansen EH. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv. 2009;74:710–717.
- Vanzetto G, Berger-Coz E, Barone-Rochette G, Chavanon O, Bouvaist H, Hacini R, Blin D, Machecourt J. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009;35:250–254.
- Nishiguchi T, Tanaka A, Ozaki Y, Taruya A, Fukuda S, Taguchi H, Iwaguro T, Ueno S, Okumoto Y, Akasaka T. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J. Acute Cardiovasc Care. 2016;5:263–270.
- Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A, Roth A. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129:1695–1702.
- Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, Naderi S, Shah S, Thaler DE, Tweet MS, Wood MJ; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation. 2018 May 8;137(19):e523-e557.