Gregory Ducrocq, Thomas R.D. Shaw, Habib Gamra, Peter C. Block, Neil R. Grubb, Carlos E. Ruiz, Alec Vahanian
Updated on November 22, 2022
Transcatheter mitral valve implantation (TMVI) techniques have been intensively developed during the last years as an alternative treatment option for patients with symptomatic mitral valve (MV) disease who are ineligible or at high risk for conventional open-heart surgery. In contrast to transcatheter aortic valve implantation, the interventional treatment of MV disease requires a more versatile approach due to the various etiologies of valvular dysfunction and the broad anatomical spectrum. The high level of complexity also makes technical developments and system engineering particularly challenging.
MV disease is the most common valvular heart disease worldwide, with primary (degenerative) and secondary (functional) regurgitation being the most common etiologies in developed countries and rheumatic heart disease in most other geographies. Left untreated, MV disease is linked to substantially increased morbidity and mortality. Treatment has long been exclusively based on surgical procedures such as valve repair or replacement. The limited applicability of open-heart surgery linked to high morbidity and mortality in elderly patients, especially those with impaired left ventricular function, has created a large therapeutic gap since up to 50% of the patients with MV disease are not referred for an intervention and remained untreated. , To improve patient care, transcatheter-based treatment options inspired by surgical techniques have been developed. Transcatheter edge-to-edge repair (TEER) is currently the most widely adopted one and is established as a safe and efficacious treatment option in both primary and secondary mitral regurgitation (MR). , , , Other technologies, including chordal repair and annuloplasty, are emerging and will further enrich the toolbox of transcatheter MV repair. , ,
Despite the continuous expansion of indications and treatment of more complex anatomies using mitral TEER, some patients with MR remain unsuitable for the procedure (e.g., leaflet thickening, heavy calcifications, shortened posterior leaflets, and retracted leaflets) and in others a suboptimal result can be anticipated in the presence of signs of anatomical complexity, . Since residual or recurrent MR affect outcomes and survival , , there is a need for complementary and more reproducible percutaneous therapy.
TMVI holds the potential to overcome these limitations through standardization (“one valve fits all”) and complete and sustained abolishment of MR. Additional advantages include the possibility to treat mixed MV disease (regurgitation and stenosis with elevated baseline gradients) including patients with mitral annular calcification (MAC), failed rings, as well as after previous clip implantation. Favorable results have been reported in these different scenarios, however, many questions remain, such as optimal patient selection, device choice, antithrombotic treatment, as well as the management of the risk of left ventricular outflow tract obstruction.
The mitral valve apparatus is a complex system involving several structures and interacting with the left ventricle (LV), atrium, and aortic valve. The D-shaped MV annulus has a dynamic saddle morphology, which varies throughout the cardiac cycle and with changes in hemodynamic status. Therefore, many dedicated TMVI devices have a dual stent design, where the outer stent interacts with the dynamic annulus, while the inner stent holds the valve leaflets.
LVOT obstruction can induce severe hemodynamic instability and is a potential life-threatening complication during TMVI procedures. The angle between the aortic and the mitral annulus, and LV dimensions are the main predictors of LVOT obstruction following TMVI, but also LV function and the presence of a septal bulge have to be taken into account. Dedicated imaging, as well as three-dimensional valve reconstruction by computed tomography (CT) represent the cornerstones of prevention. End-systolic virtual valve simulation of different devices and sizes predicts the expected minimal Neo-LVOT area and identifies patients at risk of acute obstruction (Figure 1). Depending on the procedure and valve used a minimal neo-LVOT area of 180-250mm2 is necessary. The risk of LVOT obstruction can vary according to the device design, and whether the anterior MV leaflet is secured or grasped by the prosthesis.
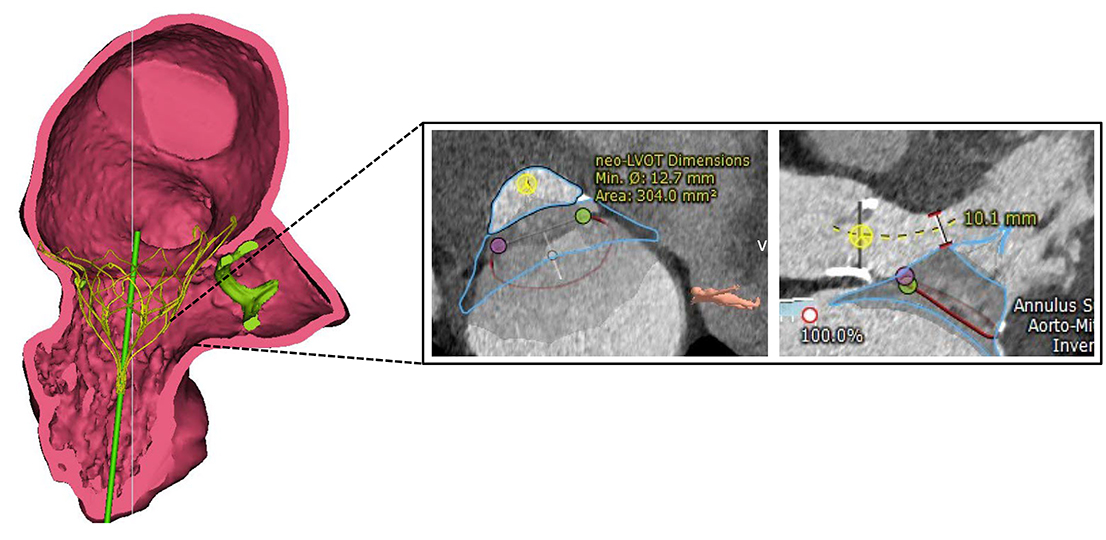
Three-dimensional valve simulation using computed tomography. Virtual implantation of a Tendyne prosthesis (Abbott) with measurement of the anticipated Neo-LVOT (left ventricular outflow tract) during systole.
Several considerations regarding device design (low profile valves, fixation/grasping of the anterior leaflet, or supra-annular valve configuration) are being evaluated to minimize this risk.
The anterior mitral leaflet (AML) has a large triangular shape and separates the LV inflow from the LV outflow. Whilst systematically removed in surgical replacement procedures, it may induce left ventricular outflow tract (LVOT) obstruction when mobilized towards the interventricular septum by implantation of a transcatheter valve. Leaflet modification techniques like intentional laceration or cutting of the AML by focused radiofrequency energy or using surgical scissors inserted through the transpical access have been proposed as possible strategies to prevent LVOT obstruction. , LAMPOON has been used with favourable results in conjunction with valve-in-ring or valve-in-MAC interventions , as well as immediately before transpical TMVI. ,
Alcohol septal ablation represents another strategy to prevent LVOT obstruction, which can lead to an increase in the Neo-LVOT area of about 110mm2. However, the procedure is linked to non-negligeable risk of peri-procedural complications such as pacemaker implantation (16%) or cardiogenic schock and should be restricted to well-selected cases. Both approaches may also be used as a bailout strategy in cases of acute LVOT obstruction after TMVI. ,
Another important consideration is related to the access site. Especially in chronic MR, the annulus can be heavily dilated, and large devices are required to obtain adequate fixation and sealing. Therefore, large bore delivery systems (≥24 Fr) are often required. Accordingly, first-generation TMVI devices were based on a transapical approach that has the advantage to allow ideal coaxiality to the MV apparatus, but necessitates a surgical cut and may lead to further LV impairment in secondary MR patients.
The less-invasive and more promising transfemoral/transseptal access remains challenging given the angulation when penetrating the left atrium through the interatrial septum. Engineering solutions to overcome these difficulties (mainly an additional plane of catheter steerability) have been implemented in the latest device iterations, although none of them is commercially available yet. It can be expected that with the development of smaller device profiles and more flexible delivery catheters, the transseptal approach may become the default strategy for TMVI.
Stable positioning and reliable fixation represent major concerns for TMVI in the native MV apparatus. Safe anchoring of the prosthesis to prevent displacement or migration is difficult to achieve given the lack of heavy annular calcification in most patients, making fixation by the sole radial force unlikely to succeed. Moreover, excessive annulus apposition may increase the risk of compression and damage to adjacent structures such as the LVOT, the inteventricular septum, the conduction system, the coronary sinus, the left circumflex artery, and the aortic root. Additional fixation elements are required to ensure proper anchoring to the valve leaflets, the subvalvular apparatus, or the LV apex. Preventing perivalvular leakage (PVL) by complete sealing is especially important in the mitral position due to a relevant risk of hemolysis.
The anatomical variability introduces further challenges like in patients with Barlow’s disease who present with excess tissue and multisegmented prolapses, which may prevent optimal fixation and increase the risk of LVOT obstruction. Asymmetric calcification, in particular when involving the leaflets, often requires predilation of the valve to avoid increased post-procedural gradients and may lead to paravalvular leakage.
There are numerous dedicated TMVI systems under development, while only one has been approved for commercial use in Europe so far (Figure 2). Common to all systems is the implantation under general anesthesia, using echocardiographic and fluoroscopic guidance for access, delivery, valve positioning, and evaluation of the post-procedural result. With few exceptions, rapid pacing is usually not required for TMVI.
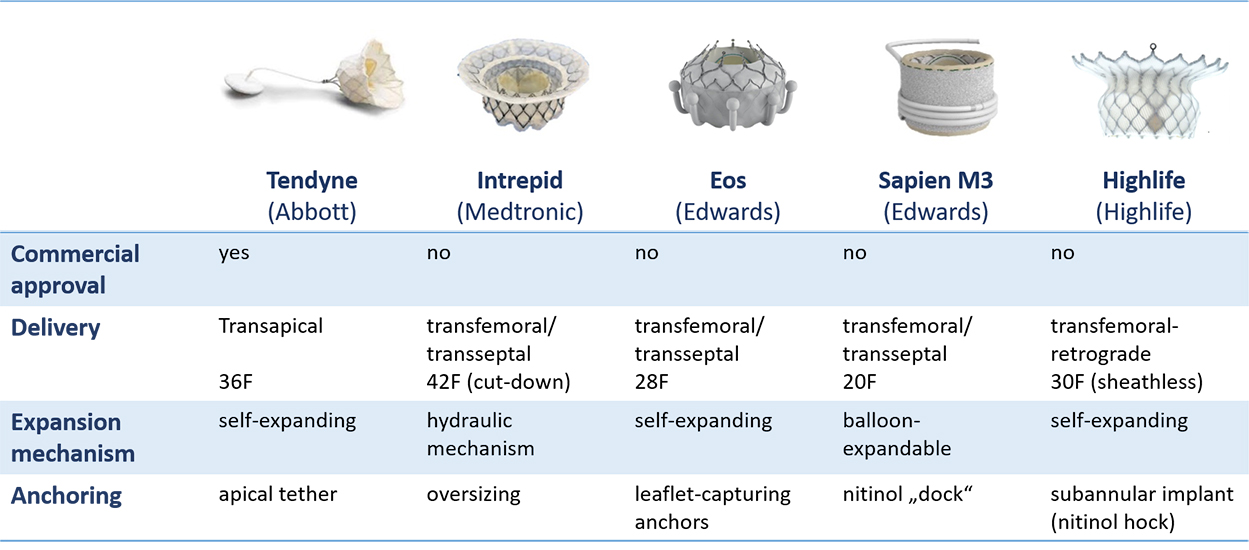
Approved and emerging devices for transcatheter mitral valve implantation (TMVI) in the native mitral valve.
The Tendyne Mitral Valve System (Abbott Vascular, Roseville, Minnesota, USA) is a dedicated transapical MV implantation system that was the first and so far the only device to obtain commercial approval in Europe (CE mark) in January 2020 for use in patients with clinically relevant MR who are ineligible for open-heart surgery. The prosthesis consists of a trileaflet porcine pericardial valve prosthesis sutured into a circular double nitinol frame (nickel-titanium alloy), that has self-expanding properties and is radiopaque. The inner stent carries the valve and is sutured to an outer stent that is covered by porcine pericardium with a polyethylene terephthalate (PET) fabric cuff to improve sealing within the native annulus. The outer stent is D-shaped and the cuff is raised against the aortic-mitral continuity to enable stable positioning of the valve. The whole prosthesis is connected to a braided fiber tether made of ultra-high molecular weight polyethylene, which is designed to stabilize the valve by passing through the left ventricular apex where it is secured to an external pad by a counter-pull principle. The pad button is covered by PET fabric, designed to promote ingrowth. Unlocking and relocking of the pin to adjust the tension on the tether is possible, even several weeks after the procedure. The amount of tension applied to the tether is calculated individually according to the systemic pressure conditions. Different valve profiles (standard and low) with different valve projection into the LVOT and multiple sizes are available, chosen according to the individual anatomy assessed by multi-slice CT and confirmed by intraprocedural three-dimensional transoesophageal echocardiography (TEE). A case example is depicted in Figure 3.
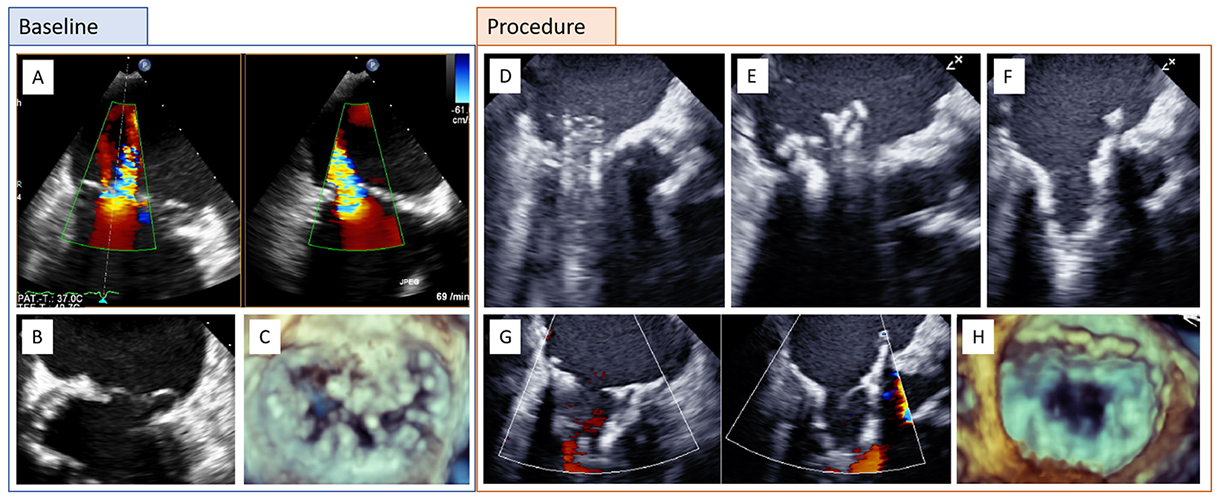
Clinical case of a patient with complex primary mitral regurgitation (MR) treated by transcatheter mitral valve implantation (TMVI) with a Tendyne bioprosthesis. A) Baseline transesophageal echocardiography (TEE color Doppler; biplane) with severe MR. B) Bileaflet prolapse and annular calcification. C) Three-dimensional (3D) echocardiography. D) TMVI procedure with advancement of the delivery catheter into the left atrium and E-F) stepwise deployment of the Tendyne prosthesis. G-H) Assessment of the final result by biplane TEE with color Doppler and 3D.
The procedure is performed using a left lateral mini-thoracotomy for transapical access. A soft wire is inserted and a balloon-tipped catheter is advanced into the left atrium to exclude entrapment in the subvalvular apparatus. A 34 Fr sheath containing the loaded bioprosthesis is then advanced over the soft wire into the left atrium. This is followed by a step-by-step deployment of the valve under TEE guiding. The prosthesis can be repositioned and retrieved, i.e. in case of left ventricular outflow tract (LVOT) obstruction. Shifting the tether slightly posterolateral rotating the valve laterally have been suggested as measures to increase the neo-LVOT, while valve alignment remains intact.
The early feasibility study included 100 selected patients at high or prohibitive surgical risk (mean STS score 7.8±5.7%) with mainly secondary MR. Despite the high-risk profile of the patient population, procedural safety and technical success were high, and 1-year survival was 72%. , Implant success was achieved in 97% of cases while three implants were abandoned or retrieved. A total of nine device-related adverse events were reported (three hemolysis and six thromboses). Symptom relief was observed in the majority of patients (New York Heart Association [NYHA] functional class I or II in 89% at 1-year follow-up), as well as a significant improvement in 6-minute walking distance and quality of life score. Durable echocardiographic results have been reported: almost all patients had none or mild residual MR at early and 2-year follow-up. However, mortality at 2 years was high (41%), which probably relates to the inclusion of a majority of patients with secondary MR, along with the use of the transapical access in this overall elderly population with LV dysfunction. Currently, the commercial use of the Tendyne valve in Europe has shifted towards treatment of a majority of primary MR patients with preserved LV function.
Real-world data from a multicentre registry showed similar improvement in MR severity and symptomatic burden but also highlighted the risk of the procedure with a 30-day mortality of 13% in a multimorbid patient population. Notably, technical and procedural success did not differ significantly in patients treated with an on- or off-label indication, potentially opening the door towards expanded indications. Compared to TEER, more frequent LV remodelling and RV function improvement were observed, probably related to a more effective MR reduction.
Upcoming data are expected from longer-term follow-up of the above-mentioned single-arm study, as well as the real-world registry. The SUMMIT trial is investigating TMVI with the Tendyne system versus TEER in MR patients, as well as MAC patients and non-repairable patients in a single-arm design.
The Intrepid (Medtronic, Minneapolis, Minnesota, USA) has a dual stent design with an outer stent engaging with the annulus (with current sizes 42 and 48mm) and providing sealing, and a circular inner stent accommodating the 27 mm tricuspid bovine pericardial valve. Anchoring is achieved mainly by oversizing (10-30% recommended) and further supporter by frictional elements and finally tissue ingrowth.
While the first generation was dependent on a transapical approach, the newer device generation can also be delivered via the transfemoral/transseptal access. Depending on the valve size, the femoral vein requires dilatation up to 42F, which has been usually achieved using surgical cut-down so far. After a standard transseptal puncture, the dedicated sheath is advanced into the left atrium followed by the delivery catheter. A flexible brim is deployed in the left atrium and positioned in the mitral plane, where the device is implanted under a short period of rapid pacing using a hydraulic mechanism. The valve can be recaptured by a flow reverser until the fixation ring is expanded and the device finally released. There is no need for rotational alignment or attempts of leaflet attachment. The system accommodates tilt and lateral misalignment.
In the early Intrepid Global Pilot Study 50 patients (72% secondary MR, mean STS score 6.4±5.5%) were treated via the transapical approach. The device success rate was 98% without reported device malfunction/thrombosis. At 30 days, mortality was 14% and rehospitalization for heart failure 8%. MR after a median follow-up duration of 173 days was mild in all patients with correpsonding symptoms and quality of life improvement. More favorable results were shown for the transseptal access in the early feasibility study, which enrolled fifteen patients (67% primary MR). Periprocedural complications occurred (six access site bleedings, one conversion to surgery, and eleven iatrogenic atrial septal defect closures), but at a 30-day follow-up, there was no death, stroke, or re-intervention.
The single-arm APOLLO study is underway to investigate the system in a larger patient cohort with mixed etiology, also including a separate study arm for MAC patients.
The Eos system (Edwards Lifesciences, Irvine, CA, USA), previously named CardiAQ and Evoque, consists of a trileaflet bovine pericardial valve mounted on a circular, self-expanding nitinol frame, covered with a polyester skirt to minimize paravalvular leak. Fixation is provided by 9 circular anchors that capture the native leaflets and chordae. It is currently available in two sizes (44 and 48mm). Both transfemoral and transapical delivery systems have been developed, but the system is now implanted exclusively via the transfemoral-transseptal approach.
The 28 F delivery system enables motion in three planes, facilitating coaxial alignment with the MV. A pre-curved guidewire is placed in the LV apex over which the delivery system is advanced. After positioning in the MV annulus, the outer capsule is retracted, releasing the ventricular portion of the prosthesis. The capture of the leaflets is carefully monitored by TEE multiplanar reconstruction with the depiction of every single anchor. Finally, the atrial portion with the sealing skirt is deployed. The latest iteration of this self-expanding valve is recapturable.
The first-in-human experience in 14 patients (mean STS score 4.6%, mainly mixed MR etiology) using the previous valve design demonstrated high technical success with one conversion to open-heart surgery due to severe PVL, and efficient MR reduction to mild or less in all patients at discharge and 30-day follow-up. Two patients underwent PVL closure, and one alcohol septal ablation for LVOT obstruction during follow-up. Symptomatic improvement to NYHA class II or less was observed in 82% of the patients.
An early feasibility study is enrolling in the US.
The Sapien M3 (Edwards Lifesciences, Irvine, CA, USA) is a balloon-expandable valve with 3 bovine leaflets identical to the Sapien 3 aortic valve with an additional external cloth covering the entire outer surface. It is implanted from transfemoral over a 20F delivery system. Before the implantation of the valve prosthesis, an expandable nitinol “dock” is placed by encircling the chordae and enables subvalvular fixation for the valve. This step requires accurate echocardiographic guidance and has a relevant impact on the procedure complexity and duration. A further potential disadvantage of the system is the implantation of a valve that has been not designed for the mitral annulus and therefore less able to accomadate complex anatomy, in particular severe calcifications.
The procedure has some similarities with a valve-in-valve implantation in failed surgical bioprosthesis with the relevant difference of immobilizing the anterior leaflet and therefore reducing the risk of LVOT obstruction. The valve is implanted into the “dock” under rapid pacing.
A first-in-human experience in ten patients with mixed aetiologies showed technical success in 90% of cases, and residual MR was less than trivial in all patients with a mean transvalvular gradient of 2 mmHg. There was no major adverse clinical event at 30 days of follow-up except for one patient with a PVL, which was treated with a closure device.
The early feasibility ENCIRCLE trial is currently recruiting patients in a single-arm study design.
The HighLife system (Highlife SAS, Irvine, California, USA) is a two-component system. The subannular implant consists of a polymer tube covered with polyester holding a nitinol hook. It is delivered through the retrograde transfemoral arterial access through the aortic valve, creating a closed loop with a fixed perimeter around the native valve leaflets and chordae. The self-expanding valve prosthesis, composed of a three-leaflet bovine pericardium valve, is implanted with a sheathless 30F system into the subannular implant ensuring safe anchoring. Given the final interaction between the valve prosthesis and subannular implant, the system does not require co-axial or rotational positioning. The subannular implant secures the anterior mitral valve leaflet away from the left ventricular outflow tract and may reduce the risk of LVOT obstruction.
An interim analysis of the first 30 patients of the early feasibility study reported acceptable technical success with uneventful implantation in 27 patients. Particular care should be taken to not damage the aortic valve during subvalvular looping. Five patients died within one year after the procedure. MR was none/trace in 75%, and mild in the other patients at 30 days.
The HighLife Transcatheter Mitral Valve Replacement System Study is currenly enrolling patients towards CE mark approval while an Early Feasibility and Safety Study is currently underway in the United States using the Clarity valve that has a minimal skirt with open cells to further minimize the risks of left ventricular tract obstruction. Over 80 patients have been treated worldwide using the transseptal delivery system.
The Cardiovalve system (Valtech Cardio Ltd, Or Yehuda, Israel) features a self-expanding pericardial bovine valve mounted on a nitinol frame, specifically designed to be delivered through a transfemoral transseptal approach. It has a multi-steerable catheter for coaxial implantation. Currently, three sizes are available covering annular sizes up to 53mm. Data about the first five patients of the early feasibility study AHEAD have been reported, showing efficient MR reduction, and a high 30-day mortality rate of 60%.
The Cephea system (Abbott, Menlo Park, California, USA) is a self-expanding nitinol double-disc device, of which one is positioned on the floor of the left atrium and the other on a subannular level. This design ensures anchoring independent from anatomical variations of the annulus. The low-profile device is delivered through transfemoral-transseptal access and is fully repositionable and recapturable. It is currently available in one size (36mm). Early reports of four patients showed technical success, MR resolution, and functional improvement in all patients. The early feasibility study is currently enrolling.
The Innovalve (Innovalve Bio Medical Ltd., Tel Hashomer – Ramat Gan, Isreal) is a transseptal system using central wrapping of the native mitral valve tissue by rotating opened arms between the chords for anchoring and prevention of LVOT obstruction before valve deployment. The TWIST trial is currently recruiting patients with primary or secondary MR.
Altavalve (4C Medical Technologies, Minneapolis, Minnesota) is a self-expanding, spherical ball-shaped, nitinol device that is anchored only by atrial fixation and holds a three-leaflet bovine valve. First transfemoral-transseptal cases have been reported. However, the supra-annular design may induce flow disturbances with increased thrombogenicity. The early feasibility study is underway.
Despite major progress achieved in MV surgery over the past few decades, 20% to 35% of patients require redo surgery or intervention due to degeneration of the prosthesis or disease progression during the first 10 years after MV replacement or repair. Redo surgery may be associated with significant early mortality (5-12%), especially in elderly patients with a high burden of comorbidities. ,
The treatment of failed surgical MV bioprostheses with a transcatheter heart valve device (THV: used in transcatheter aortic valve implantation procedures) has emerged as an alternative to redo surgery in patients at increased surgical risk. During the last few years, this off-label approach has been adapted for failed MV prostheses or rings, mainly using the Sapien aortic valve (Edwards Lifesciences, Irvine, CA, USA). ,
Surgical intervention in patients with severe mitral annular calcification (MAC) is technically challenging and poses an increased risk of rupture of the mitral annulus or the aorto-mitral continuity, as well as debris embolism.
Transapical access was the access route of choice in early ViV, ViR, and ViMAC procedures, whereas the transfemoral/transseptal approach is the default strategy nowadays. The procedure is performed under general anesthesia using mainly fluoroscopic guidance, though transseptal puncture and assessment of the final deployment are usually facilitated by TEE guidance. A temporary pacemaker for rapid pacing may be placed in the right ventricle.
Using transvenous femoral access, a steerable catheter (e.g., Agilis, St. Jude Medical, St. Paul, MN, USA) is directed across the MV. A 5 Fr Multipurpose catheter (e.g., Cook Medical, Bloomington, IN, USA) is then advanced on a standard guidewire over which an SAFARI² Extra Small guidewire (Boston Scientific, Marlborough, MA, USA) with a “J” curve at the end is placed in the apex of the left ventricle (Figure 4 A). The atrial septum is dilated using a 12-14 mm peripheral balloon (Figure 4 B). The THV device , mounted upside down on the catheter (as compared to the aortic position), is advanced through the atrial septum and positioned in the MV plane using maximal flexion of the catheter in a projection perpendicular to the plane of the bioprosthesis/ring/MAC (Figure 4 C-E). This is usually facilitated by the radiopaque ring or bioprosthesis frame. Few bioprostheses have only a few or no radiopaque markers, in which cases positioning has to be guided by echocardiography. Implantation is performed by slow balloon inflation under rapid ventricular pacing (160-200 bpm) to allow correction and perfect adjustment of the position (Figure 4 F).
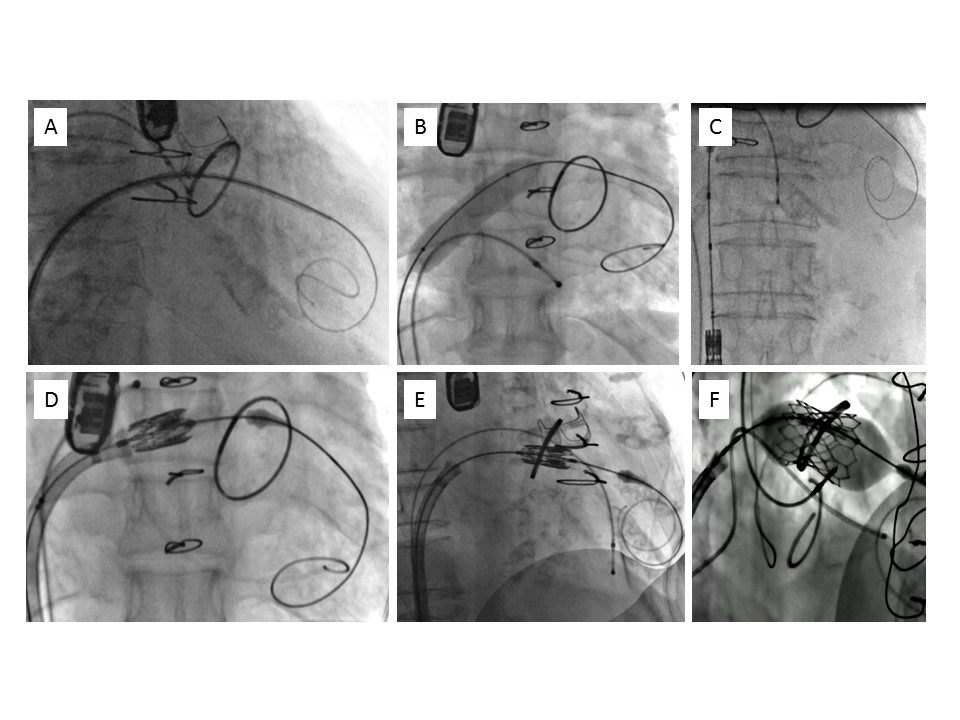
Transvenous, transseptal mitral valve-in-ring implantation. A) Guidewire placement at the apex of the left ventricle. B) Dilatation of the intratrial septum. C) Loading of the valve prosthesis in the inferior vena cava. D) Crossing of the interatrial septum using delivery catheter flex. E) Positioning in the mitral ring in a perpendicular plane. F) Valve deployment by progressive balloon inflation under rapid pacing.
Crossing the calcified annulus may be more challenging than in ViV or ViR procedures due to the presence of calcium excrescences, as well as rigidity of the mitral leaflets and subvalvular apparatus. In most MAC cases, echocardiographic guidance of positioning and deployment is mandatory.
It is irremissible to have detailed information about the bioprostheses or ring that was surgically implanted before planning a ViV or ViR procedure. As there is a discrepancy between the inner stent diameters of surgical bioprostheses labeled by the manufacturers and the dimensions measured by echocardiography and computed tomography, careful evaluation should be systematically performed to choose the appropriate size of the transcatheter valve to be implanted. The Valve in Valve Digital Application (Mitral) is a very useful tool to guide the choice of optimal sizing for the most frequently used mitral bioprostheses/rings. Multimodality imaging can be used for a detailed evaluation of the individual anatomy including leaflet height. The risk of LVOT obstruction induces by the open prosthesis' or native leaflets should be taken into account and can be minimized by radiofrequency laceration, similar to TMVI in native anatomy.
In addition, for stenosed rings, sizing should take into account not only the inner ring diameter but also the adjacent valvular tissue, which is not detected by CT or fluoroscopy. The measurement of an inflated balloon during predilatation may help to assess the amount of valvular tissue and avoid implanting a too large valve. Rigid rings and the presence of open segments present further challenges in ViR procedures, where the risk of underdeployment with subsequent central or paravalvular regurgitation, high gradient, and premature degeneration should be considered.
In patients with severe MAC, sizing should be mainly guided by CT assessment of the mean diameter or perimeter in expectation of circularisation of the MV annulus after TMVI. The use of small transcatheter valves (such as a Sapien 3 23mm) should be avoided because of high transvalvular gradients. In borderline diameters, the larger valve size should be preferred to maintain safe anchoring and avoid atrial migration.
Over the last years, several registry data and retrospective cohort studies on ViV and ViR have been published. The largest experience is reported by the Valve-In-Valve International Data (VIVID) registry, including a total of 857 ViV and 222 ViR cases with a median follow-up of 492 days. Patients included had a high surgical risk profile (median STS score 8.6%), and mixed mechanism of failure (regurgitation and stenosis). The overall technical success rate was high (91%), but gradients over 5 mmHg occurred in 61% of patients. Malpositioning with the need for an additional valve in some cases, and LVOT obstruction were rare (both 3%), and occurred more frequently in the ViR group (6% vs. 2% in the ViV group). Mortality at 30 days was 6.5% and 9% for the ViV and ViR groups, respectively. Two other large contemporary registries reported similar outcomes for ViV and ViR procedures. , Both found worse outcomes for ViMAC patients with lower device success, and substantially higher complication (e.g. LVOT obstruction) and mortality rates.
The MITRAL (Mitral Implantation of TRAncatheter vaLves) trial was the first to prospectively assess outcomes after mitral ViV, ViR, and ViMAC procedures. The 1-year data of the ViV arm (30 patients, median STS score 9.4%) reported high technical success (100%) for transseptal implantation of the Sapien 3 valve. The mortality rate at 30 days in this high-risk population was low (3%) and remained unchanged over 1-year follow-up. Echocardiographic follow-up at 1 year demonstrated a reduction of MR to mild or trace in all patients with a mean MV gradient of 6.6 mmHg. Two-year data from the trial on ViV (n=30), ViR (n=30), and ViMAC (n=31) reported all-cause mortality rates of 7%, 50%, and 39%, respectively. The majority of the surviving patients improved symptomatically according to New York Heart Association class, as well as quality of life questionnaires and MR was mild or less in all patients.
A recent large meta-analysis, including more than 3000 patients from nine retrospective cohort studies, compared redo surgery to ViV for failed mitral bioprostheses. The transcatheter approach was associated with lower rates of in-hospital mortality, stroke, vascular complications, new pacemaker implantation, renal dysfunction, and severe bleeding. On the other hand, surgical redo had lower PVL rate. Valve performance, transvalvular gradients as well as 30-day and 1-year mortality did not differ.
Analog to the experiences with transcatheter aortic valve implantation, the access route has a high impact on outcome. A meta-analysis of over 2000 patients undergoing transfemoral-transseptal or transapical ViV or ViR reported lower short-term and 1-year mortality, as well as shorter hospital stay for the transseptal approach. Other clinical endpoints such as stroke, bleeding, or LVOT obstruction also favored transfemoral access but did not reach statistical significance.
Reports on other transcatheter heart valves are limited to case reports. , A recently published report on the balloon-expandable Myval valve (Meril Life Science, Vapi, India) including 64 mitral patients showed high technical success and a good safety profile.
In summary, while ViV implantations are very safe and represent a good alternative to redo open-heart surgery, ViR and ViMAC implantation remain at high risk of complication and motality, mainly because of the risk of LVOT obstruction and valve migration (Figure 5). Dedicated devices should be therefore preferred for these specific indications. Thanks to excellent performance and safety, the indication for ViV procedures may be extended to selected young lower-risk patients with the aim to delay mechanical valve implantation and long-term anticoagulation, in particular in women in childbearing age.
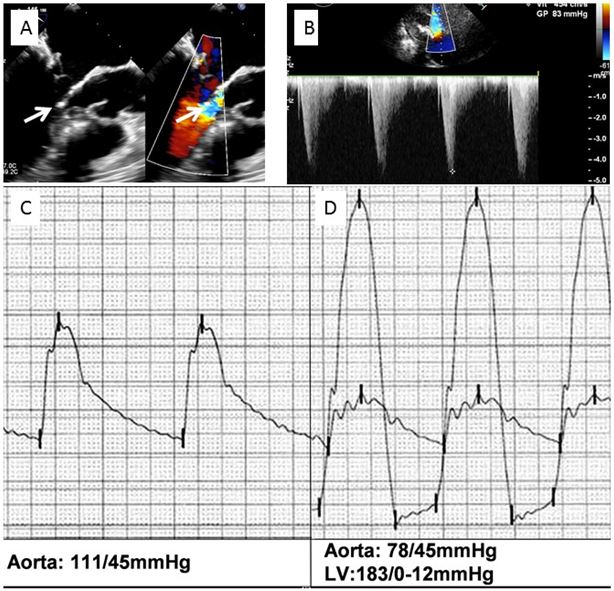
Left ventricular outflow tract obstruction after transcatheter implantation of a SAPIEN XT valve in a mitral annulus calcification. A) 2D transoesophageal echocardiography, long-axis view; contact between the subvalvular mitral apparatus and the septal bulge (left arrow) and aliasing in the left ventricular outflow tract (right arrow). B) Increased gradient in the left ventricular outflow tract. C) Aortic pressure before implantation. D) Severe hypotension with 105 mmHg ventriculo-aortic peak to peak gradient post implantation.
Patients with severe symptomatic MR who are not surgical candidates are discussed in the Heart Team and screened for anatomical feasibility of edge-to-edge repair (TEER). Mitral TEER has several advantages compared to TMVI. TEER utilizes a transfemoral approach, has limited interaction with the native MV anatomy, is safe, and facilitates fast patient recovery. Available TEER systems are well-studied, commercially approved, and have a high level of operator experience. Nevertheless, MV anatomical complexity can be a relevant limitation for TEER in particular in patients with primary MR, and in this particular patient population residual or recurrent MR is not uncommon.
MV replacement or implantation achieves predictable and durable elimination of MR. However, TMVI may be associated with a non-physiological inflow pattern, increased LV stress, loss of the normal aorto-mitral continuity, and reduction of basal LV contraction due to the fixation of the prosthesis to the mitral annulus. All this might have a detrimental effect on cardiac output, especially in patients with heart failure. Based on the surgical experience, the lifelong increase of thromboembolic or hemorrhagic events, as well as the risk prosthesis-related endocarditis, has to be borne in mind. TMVI allows the effective treatment of more complex anatomies, such as combined MV disease, calcifications, and challenging leaflet morphologies (thickening, retraction, short PML), and the use of the same device in many different anatomies (one valve for all). Notwithstanding, the screening failure rate of current TMVI devices remains high: the CHOICE-MI registry reported screening failure in two-thirds of patients, mainly due to anatomical reasons (risk for LVOT obstruction, too big or too small annular size, small LV dimensions, MAC) and an less frequently to co-morbidities or other reasons. TMVI after failed TEER facilitated by electrosurgical laceration has been proposed as a new progressive approach. Careful patient selection, including consideration of patients’ individual and anatomical aspects, plays a fundamental role in identifying those most likely to benefit from TMVI.
The choice and optimal duration of antithrombotic therapy after TMVI remains unknown and currently follows the empirical recommendations of surgical MV replacement. According to current recommendations, oral anticoagulation with a vitamin K antagonist should be installed for 3 to 6 months. In the absence of other indications, such as atrial fibrillation, it may be replaced by an antiplatelet agent. Reports of delayed valve thrombosis suggest a benefit of long-term oral anticoagulation in the absence of increased bleeding risk, especially given the lower-pressure environment of the MV. Besides vitamin K antagonists, the use of Rivaroxaban in patients with atrial fibrillation and a bioprosthetic MV has been encouraged by recent study results even if the study population was heterogenous with inclusion at variable points in time.
Transcatheter treatment options for MV repair have evolved rapidly over the last decade, though the field of TMVI has been somewhat slower with most dedicated devices still under investigation. Nevertheless, TMVI is already enriching the toolbox of percutaneous MV interventions as a feasible and safe therapeutic option for patients in whom a suboptimal result is anticipated with TEER. Multimodality imaging as well as complementary techniques such as LAMPOON can further advance TMVI planning and procedures and decrease the number of screen fails. For patients with degenerated MV bioprosthesis, ViV TMVI is the treatment of choice when the surgical risk is increased. ViR and ViMAC procedures are more hazardous and require adequate benefit/risk analysis on a case-by-case criteria.
Futures challenges of TMVI include higher safety, prevention of LVOT obstruction, treatment of a broader range of anatomies, and development and refinement of the transfemoral/transseptal approach. Upcoming results of the ongoing prospective studies will help to better understand the potential and limitations of TMVI, as well as individualize patient and device selection.
Gregory Ducrocq, Thomas R.D. Shaw, Habib Gamra, Peter C. Block, Neil R. Grubb, Carlos E. Ruiz, Alec Vahanian
Updated on November 22, 2022
Horacio Medina de Chazal, Ali Zgheib, Angelo Quagliana, Michael Chetrit, Jean Buithieu, Giuseppe Martucci, Marco Spaziano, Ali Abualsaud, Ole de Baker, Laurence Campens, Pascal Theriault-Lauzier, Jere...
Updated on November 23, 2022
Mirjam Wild, Maurizio Taramasso, Alison Duncan, Giulio Russo, Dominique Himbert, Francesco Maisano, Nicolo Piazza, Fabien Praz
Updated on November 21, 2021
Olaf W. Franzen, Ole De Backer, Mirjam Wild, Lars Sondergaard
Updated on May 13, 2021