Horacio Medina de Chazal, Ali Zgheib, Angelo Quagliana, Michael Chetrit, Jean Buithieu, Giuseppe Martucci, Marco Spaziano, Ali Abualsaud, Ole de Baker, Laurence Campens, Pascal Theriault-Lauzier, Jere...
Updated on November 23, 2022
Atrial transseptal puncture was developed to provide precise left-heart haemodynamics for the assessment of valve disease. This use was replaced by echocardiography, but it continued to be used for aortic and mitral balloon valvuloplasty and left-heart pathway ablations. Over the past decade, the role of transseptal puncture has increased greatly for atrial fibrillation ablations, transcatheter repair of mitral regurgitation, closure of some patent foramen ovales, left atrial appendage obliteration, paraprosthetic valve leak repair, positioning of left ventricular assist devices, pulmonary vein stenting, and resynchronisation therapy. This resurgence has been accompanied by new devices and variations in the classic technique. While fluoroscopy is still largely used to guide transseptal puncture, intraprocedural echocardiography has become widely used in specific interventions.
The objective of atrial transseptal puncture is the safe and effective delivery of catheterisation equipment from the right atrium (RA) to the left heart. It was introduced into clinical practice in 1959 by Ross, Morrow and Braunwald , . Its initial use was to provide precise haemodynamic measurements to help in the selection of patients for cardiac valve surgery. It allowed simultaneous measurement of pressure gradients across the mitral and aortic valves. Forward and backward indocyanine green dye curves permitted calculation of cardiac output and valvular regurgitant fraction. Technically, Manfredi described the use of a curved transseptal catheter/needle in 1956, and Cope reported the use of a straight needle in 1959 . Before the introduction of transseptal puncture, left atrial (LA) pressure was only available using more uncomfortable techniques, such as needle puncture from the suprasternal notch (the Radner technique) , via bronchoscopy , , or by a paravertebral approach . Intercostal puncture into the left ventricle could also be used for gradient recording in patients with aortic stenosis. These earlier techniques had a surprisingly good safety record , reflecting the body’s tolerance of a thin sterile needle because of the elastic recoil of the tissues.
With the progressive development of echocardiographic imaging and Doppler measurements, the use of atrial transseptal puncture declined. However, it was continued in the 1980s for mitral balloon valvotomy and antegrade aortic stenosis balloon dilatation . Since the turn of the century, there has been a major resurgence in its use. At present, by far the most common indication is left-heart electrophysiological ablations, but it is also being used for a variety of new interventional procedures, including percutaneous mitral valve repair, some patent foramen ovale (PFO) closures, left atrial appendage (LAA) occlusion, paraprosthetic valve leak repair, percutaneous valve-in-valve and valve-in-ring implantation , as well as emerging valve implantation in native annulus and finally for positioning of left ventricular assist devices within the LA. It is also very occasionally used for diagnosis (Table 1). Initially, the emphasis had been on achieving a safe transseptal puncture at the fossa ovalis, but complex interventional techniques often require a precise location of the crossing point within the atrial septum to facilitate intervention. This has led to an increasing use of echocardiographic imaging guidance in addition to fluoroscopy for these procedures, which has also increased safety.
Indications for transseptal catheterization.
AF, atrial fibrillation; ASD, atrial septal defect; AV, atrioventricular; EP, electrophysiological; LA, left atrial; LAA, left atrial appendage; LV, left ventricular; PFO, patent foramen ovale; RF, radiofrequency.
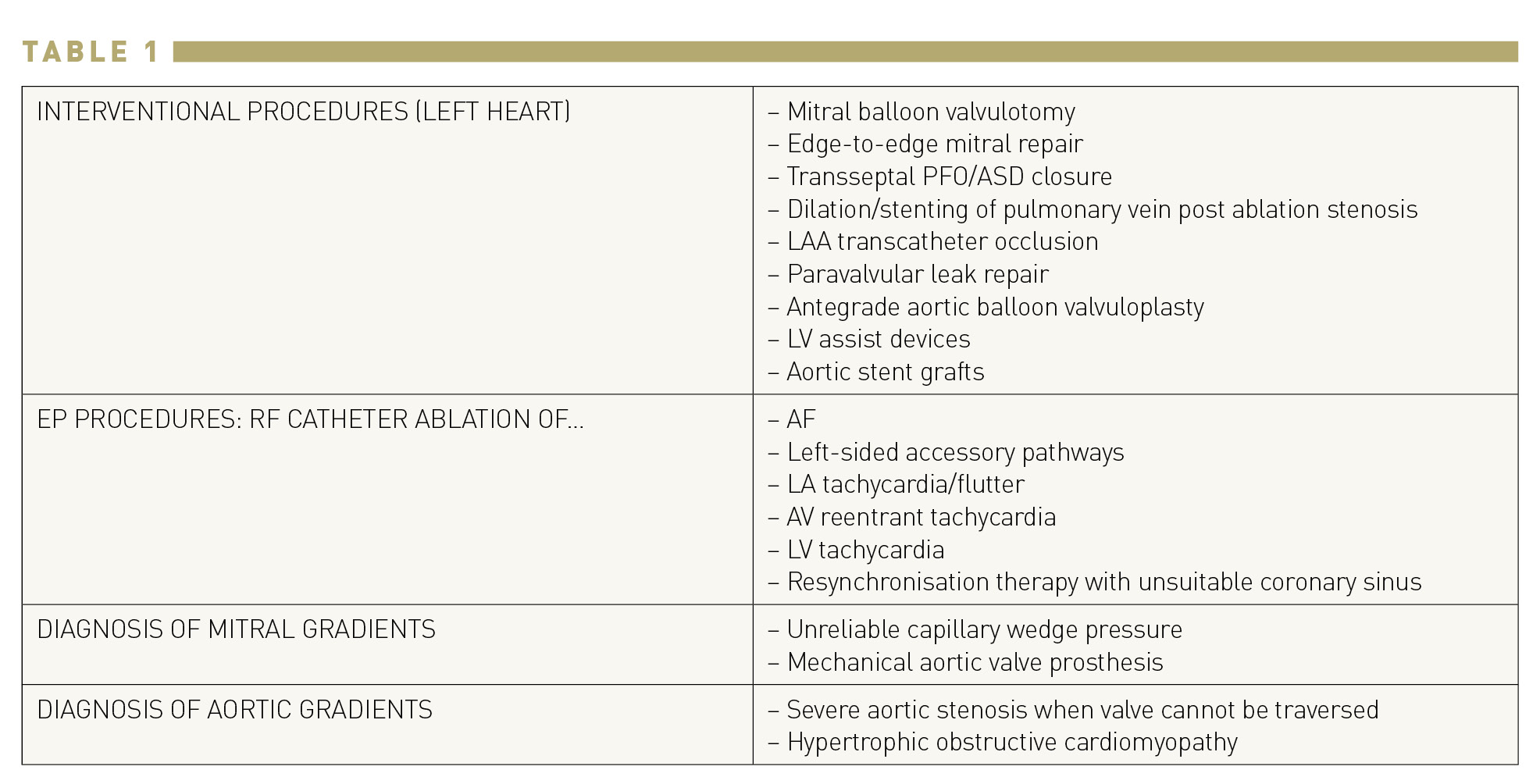
Transseptal puncture is possible because the right femoral vein is large and straight enough to allow passage of a needle that has a distal curve sufficient to turn towards the atrial septum. The proximal hub of the stainless steel needle has an arrow that corresponds to the direction of the curve (Figure 1).
Brockenbrough needle. The distal curve corresponds to the direction of the arrow at the hub.
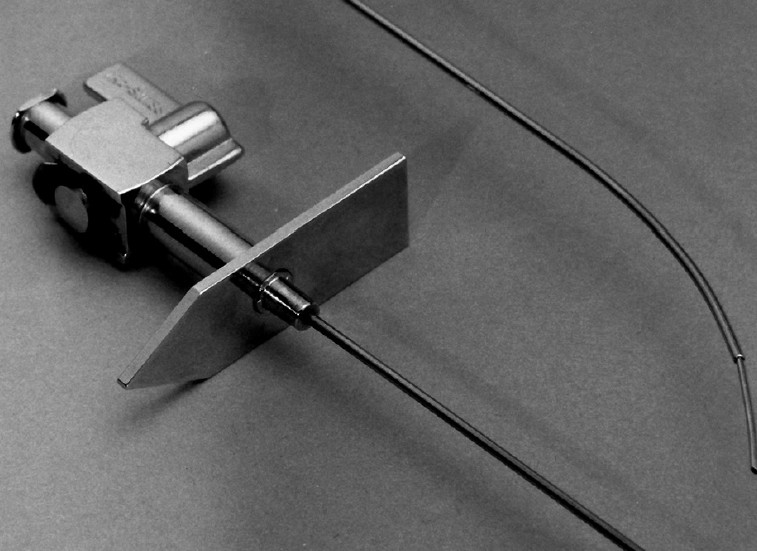
The original Ross needle was modified by Brockenbrough et al. , by reducing the calibre of the needle from 18 to 21 gauge in its distal 1.5 cm. This gave greater safety in the event of any needle puncture outside the LA cavity. Multiple types of fixed-curve sheaths are commonly used today for transseptal catheterization, including the classic Mullins sheath (Medtronic, Minneapolis, Minnesota) but also the Swartz introducer series (Abbott, Abbott Park, Illinois) (Figure 2). They have a tight-fitting outer sheath, which can be used to deliver other catheters (e.g. Swan−Ganz balloon catheters) or a 0.035 wire to the left heart. Other sheaths of various shapes and sizes have been developed more recently to allow LAA access according to specific indications .
Brockenbrough needle within the Mullins catheter (above) and within the Swartz catheter (below).
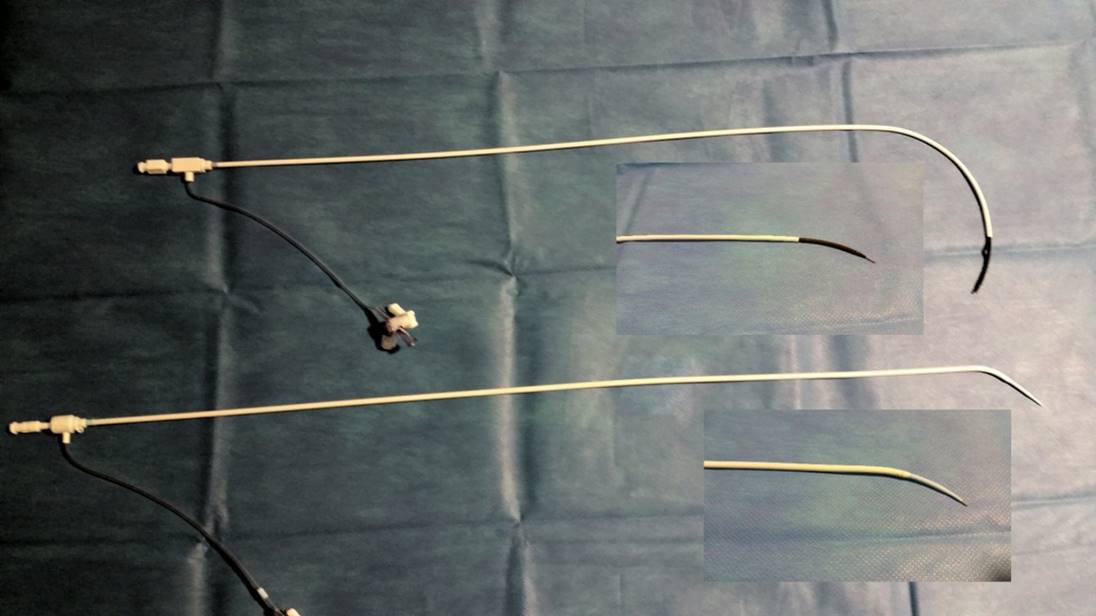
The needle is not a surgical instrument, and its long lumen means that adequate cleaning cannot be assured, so re-sterilisation is not recommended. Several manufacturers now produce transseptal sets, usually with only minor variations in design . The “historical” Brockenbrough needle (Medtronic, Minneapolis, Minnesota) presented in Figure 1 is available in one size. However, other manufacturers have developed transseptal needles with different angulations, such as the BRK series (St. Jude Medical, St Paul, Minnesota, USA) (Figure 3). “ES” extra-sharp versions have also been developed to facilitate septum crossing. Paediatric versions are also marketed.
Transseptal needles with different angulations.
The Endrys transseptal coaxial double-needle system (Cook Europe, Bjaeverskov, Denmark) is used by some, particularly when there is a small LA, and the very thin inner needle is believed to improve safety (Figure 4).
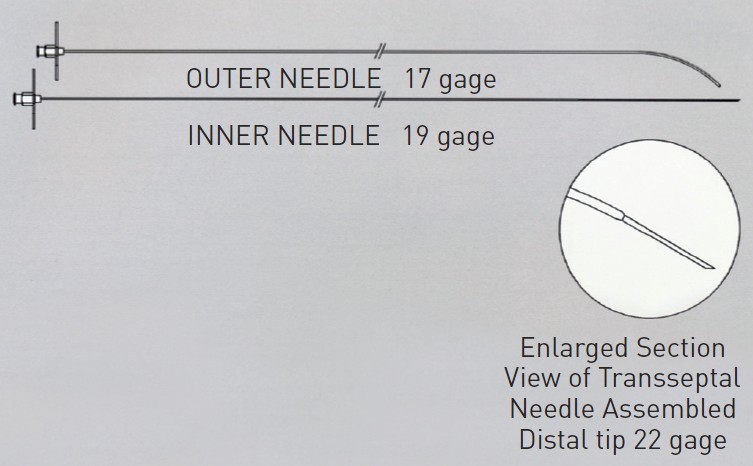
Endrys needle and inner needle.
Similarly, the SafeSept™ (Pressure Products, San Pedro, California) includes a 0.014” wire, which is advanced through the needle lumen. It is designed with a very sharp tip that can penetrate the septum more easily than the needle bevel. As soon as this wire enters the LA it takes up a J-shape to avoid touching the posterior LA wall .
The NRG transseptal needle (Baylis Medical, Montreal, Canada) system uses radiofrequency. The needle is connected to a generator (Figure 5). When positioned at an optimal location, radiofrequency is applied, facilitating crossing of the needle across the septum. The tip of the needle is blunt to allow delivery of radiofrequency to the septum. The NRG needle is available in different-shaped curves. The radiofrequency energy creates a hole in the atrial septum of equivalent size to that created by a needle. The needle delivers 5 W of energy for 2−5 seconds and usually perforates the atrial septum after 1−4 pulses. The advantage of the NRG transseptal needle is that little forward pressure is needed. Thus, the radiofrequency energy can create a hole in septa that are thick, scarred or calcified, and might even be used in those with previous surgical patching .
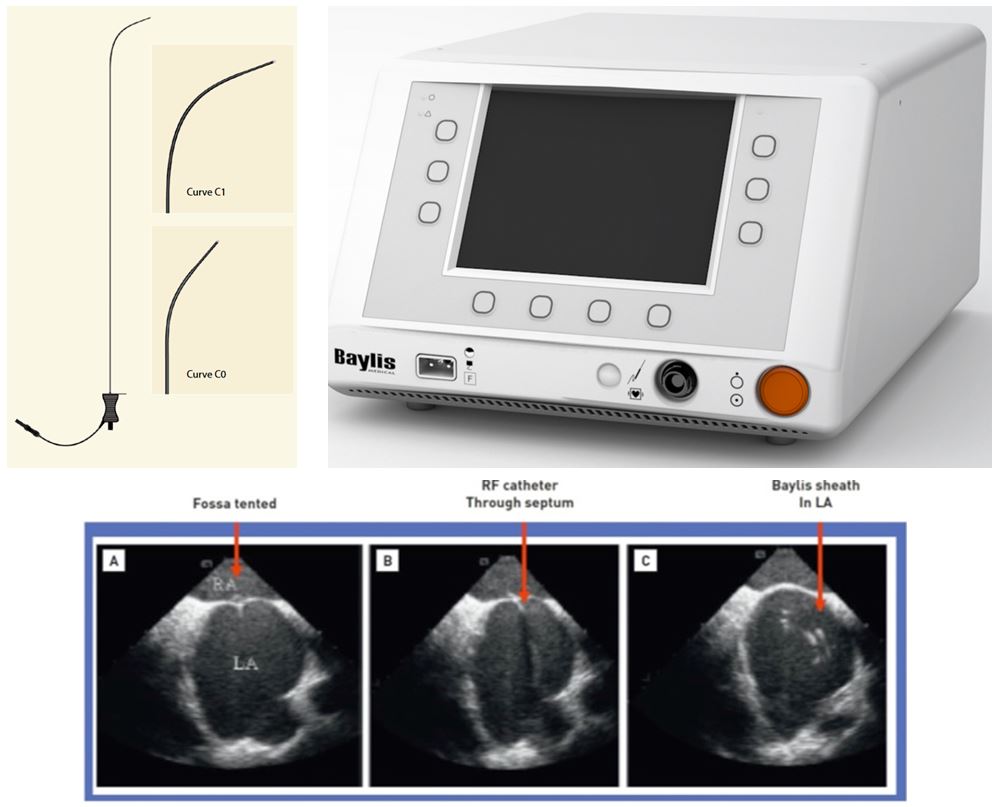
NRG transseptal needle (Baylis Medical, Montreal, Canada), (upper left) and generator (upper right). Lower panel: Puncturing the septum with the Baylis curved tip catheter: (A) fossa tented; (B) radiofrequency catheter through septum; and (C) Baylis sheath in left atrium (LA).
The VersaCross (Baylis Medical, Montreal, Canada) device comprises a wire placed into a dedicated catheter. The catheter can be easily reshaped by the operator to have a more or less pronounced curve. The wire is connected to a radiofrequency generator. When the catheter is positioned on the septum at an optimal location, the wire is advanced outside the catheter for a few millimetres. In this position, the wire remains straight. Radiofrequency energy is applied to allow the wire to cross the septum. The wire is then further advanced, and takes on a pigtail shape that allows the catheter to be advanced into the left atrium (Figure 6).
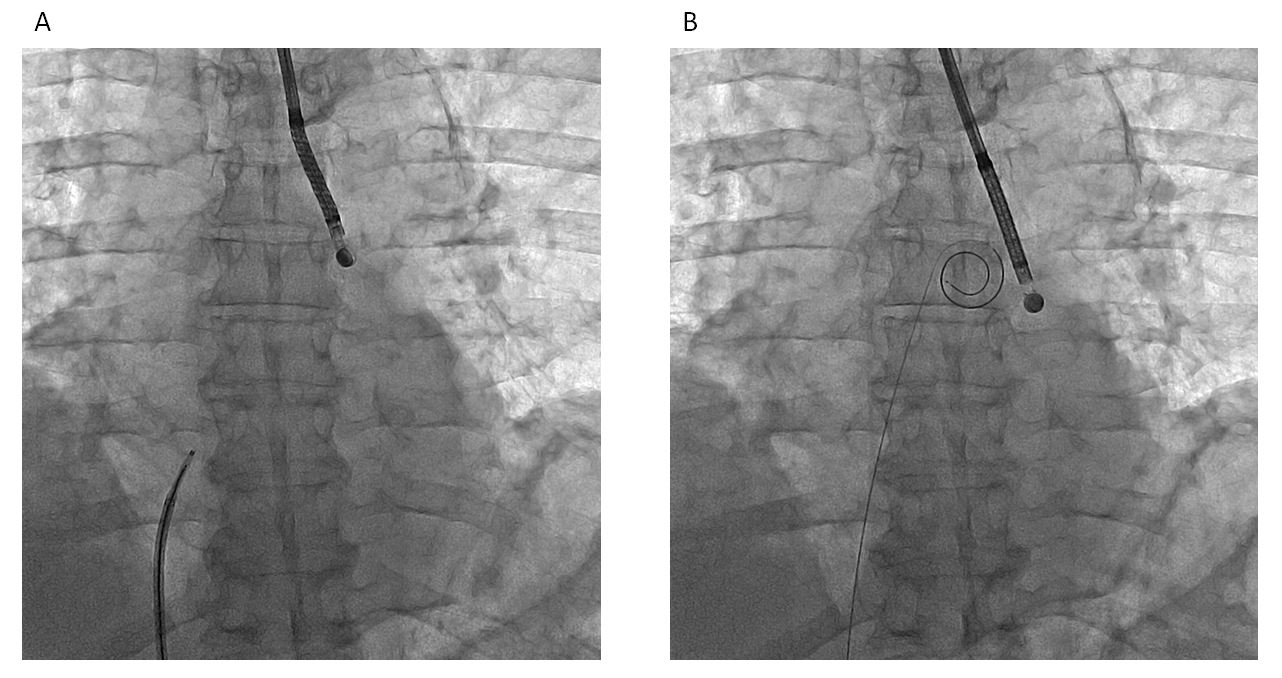
VersaCross radiofrequency-wire based platform (Baylis Medical, Montreal, Canada). (A) Wire in the catheter (left) and wire in pigtail shape after crossing the septum (right).
The following are usually listed as contraindications to transseptal puncture:
Of these, the only absolute contraindications to transseptal puncture are a thrombus located at the septum and an atrial myxoma or other tumour arising from the septum. The other conditions make transseptal puncture more difficult but are regularly dealt with by experienced operators. The presence of structural abnormalities makes additional information from intraprocedural echocardiography very helpful.
Transoesophageal echocardiography (TOE) is indicated before transseptal puncture when there is structural heart disease or chronic atrial fibrillation (AF) to look for atrial thrombus. The presence of any LA thrombus always calls for great caution because of the danger of causing embolism. Normally any left-heart procedure is contraindicated when any atrial thrombus is present. Most atrial thrombi will resolve over 3−6 months of intensive anticoagulation (aiming for an international normalised ratio [INR] of 3.5−4.5). The performance of the procedure can therefore be deferred and performed safely if repeat TOE confirms the thrombus has resolved. However, transseptal intervention can be indicated in patients who are candidates for urgent life-saving intervention but not surgery. Mitral balloon valvotomy has been successfully performed when the thrombus is located well within the LAA and is non-mobile. In this case, some operators use cerebral protection , .
Finally, a thrombus-trapping technique has been described in LAA occlusion and seems feasible in selected cases .
Transseptal puncture through an intra-atrial patch is a safe procedure . Successful puncture in the presence of an Amplatzer septal occluder has also been reported .
When the heart is displaced by pulmonary changes, the inferior vena cava (IVC) does not follow a normal path, and the alignment of the catheter/needle and the LA cavity may be much altered: additional information echocardiography is then particularly helpful. Some operators also use contrast injection in this case.
With azygous continuation of the IVC, venous drainage from the lower body does not join the inferior surface of the RA but continues to enter near the superior vena cava (SVC). This uncommon anomaly makes transseptal puncture from the IVC impossible and an SVC or transhepatic approach is required. It should be diagnosed by X-ray before the procedure. When identified during the procedure, the catheter has an unusually posterior position behind the heart (Figure 7), confirmed by venous angiography in the RA .
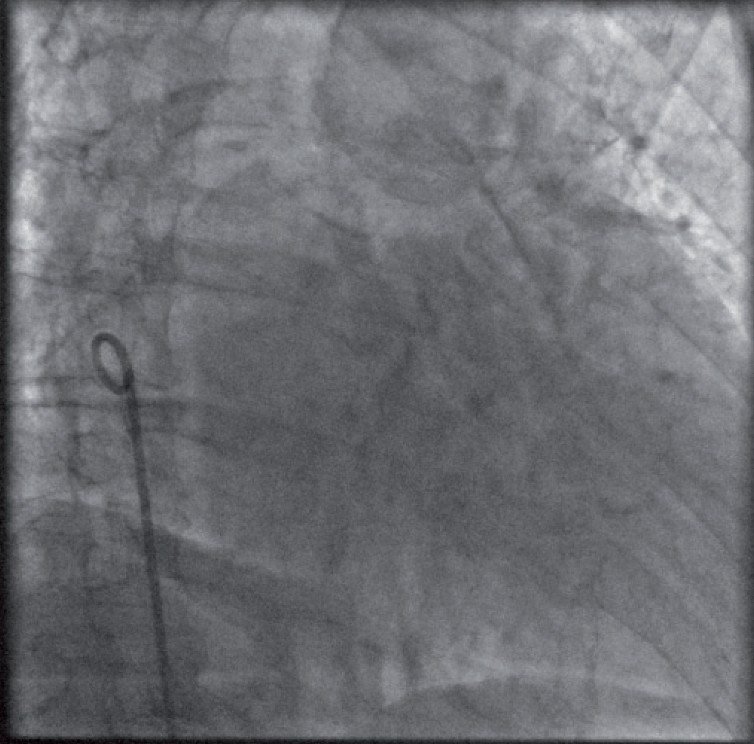
Azygous continuation of the inferior vena cava (lateral view).
Complex congenital diseases are also considered contraindicative for transseptal puncture due to the altered anatomical landmarks.
Transseptal catheterization can be performed in dextrocardia by experienced operators using left femoral access, inversion of the X-ray image and echocardiography .
Obstruction of the vena cava is a classic contraindication for femoral access, but membranous obstruction of the vena cava can be dilated. An IVC filter does not necessarily preclude transseptal puncture via the femoral vein if flow is still present in the IVC, as the equipment is sufficiently slim to pass through most models.
If there is marked osteoarthritic change in the lumbar spine, the needle curve may refuse to be advanced across the iliac crest. This can be overcome by partially withdrawing the catheter, re-advancing the needle to the point of resistance and the forward movement of the catheter then carries the needle past the obstruction.
A history of pelvic radiotherapy can dramatically increase iliac veins stiffness. In this case, the needle might not cross tortuosities.
Thoracic deformity, such as kyphoscoliosis, is a contraindication if severe. In practice, the level of acceptable deformity depends on operator experience. Echocardiographic guidance can overcome this difficulty.
Traditionally, transseptal catheterisation should be avoided, if possible, in a patient with a bleeding diathesis or an INR >1.5 (particularly in patients who have not undergone previous cardiac surgery). When intravenous heparin is used, it should be discontinued 4 hours before the procedure and can be restarted 2 hours afterwards. However, this rule has been challenged recently, when the risk of thrombosis resulting from the withdrawal of anticoagulation in lengthy procedures may be worse than that of bleeding. This is the case for transseptal puncture for AF ablation because of the long duration of the procedure, the presence of AF and the thrombotic effect of a radiofrequency lesion, and because clotting in the LA and/or on the transseptal sheath and ensuing stroke are always feared. Hence, it is now recommended for AF ablation to perform transseptal puncture and catheter ablation of AF without warfarin discontinuation and with heparin on top of vitamin K antagonists, which reduces the occurrence of periprocedural stroke and minor bleeding complications. Regarding non-vitamin K antagonist oral anticoagulants (NOACs), it is not recommended to withhold them or to hold one or two doses before AF ablation .
Similarly, In MitraClip implantation, when performed by experienced operators under TOE, it is possible to perform transseptal puncture with an INR >2 .
Except in the specific cases described above, a bolus heparin dose of 3000–5000 units or up to 100 units per kg body weight is usually used after transseptal puncture.
A transthoracic echocardiogram (TTE) gives a good and important overview of the cardiac structures and their function. The apical four-chamber view shows the thickness and curvature of the atrial septum and the size of the atrial cavities as well as abnormalities, such as atrial septum aneurysm or atrial myxoma. In addition, the presence of even a mild-to-moderate pericardial effusion should be described in the echo report to serve as a comparator if haemopericardium is suspected during the procedure.
While transthoracic views can reveal atrial thrombus, they cannot be used to exclude its presence, particularly in the atrial appendage. The performance of TOE is therefore recommended for all patients due to undergo transseptal puncture.
In specific interventions such as paravalvular leak closure or LAA occlusion, heart multislice computed tomography (MSCT) with three-dimensional reconstruction using dedicated software such as 3mensio (Pie Medical Imaging, Maastricht, the Netherlands) can help tailor the transseptal puncture to have the most direct access to the targeted heart structure (Figure 8).
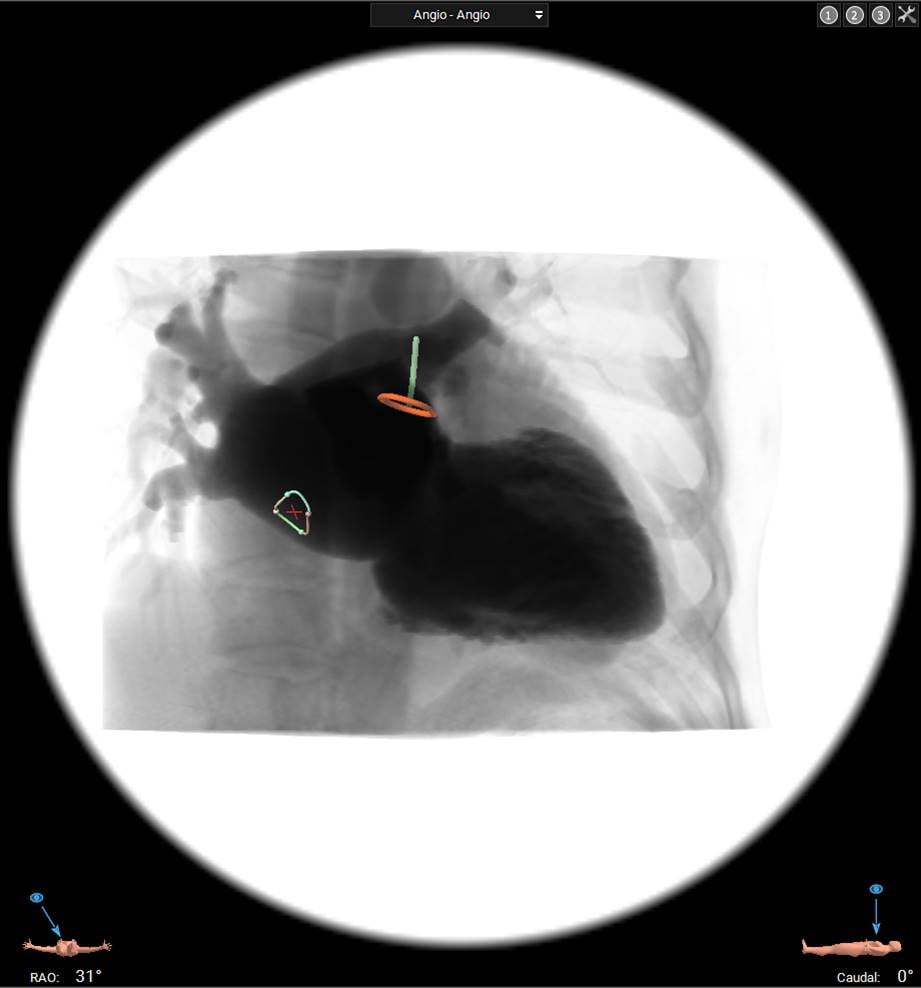
Multislice computed tomography with three-dimensional reconstruction for planning left atrial appendage occlusion. Fossa ovalis and left atrial appendage are identified to target optimal transseptal puncture. RAO, right anterior oblique.
The patient lies supine on the table unless in pulmonary oedema. A peripheral intravenous catheter is in place for drug/fluid administration. When TEE guidance is performed, general anaesthesia is usually required.
There is no single way to perform atrial transseptal puncture. In practice, most operators follow the practice of their training mentor and build up their own experience of that approach.
In the absence of echo guidance, the puncture can be undertaken using any of four fluoroscopic positions, (preferably) combining two of them.
Anteroposterior was the fluoroscopic projection used in the initial descriptions of the procedure. Many operators still prefer this projection as it is the fluoroscopic view with which they are most familiar, and it gives a good overall view of the cardiac silhouette and lung fields.
When transseptal puncture was first used, the catheter was advanced over a guidewire to the RA cavity. With the wire withdrawn, the needle was then advanced through the catheter, taking care to allow the needle to turn and twist without hindrance as it advanced to the catheter tip. The catheter/needle was then rotated towards the LA, as judged by fluoroscopy, and the needle advanced.
In 1965, Bloomfield and Sinclair-Smith described a way in which the limbic edge, the ridge where the septum secundum overlies and usually fuses with the septum primum, could be identified. They passed the catheter over a wire to the SVC. The needle was advanced to lie just within the catheter tip. In the past, the distance between the needle hub and the catheter hub when the needle was just within the catheter was noted and marked. With modern fluoroscopy equipment, this is not needed. The needle curve is directed to 4 o’clock (as seen from below) or up to 6 o’clock for a very dilated LA. The catheter/needle is then slowly withdrawn, moving medially as the RA is entered. As one drops over the limbic edge, a slight movement may be seen and/or felt, indicating that the tip has entered the fossa ovalis and a safe crossing area has been reached (Figure 9). The catheter/needle is withdrawn a little further and then advanced upwards to find a catch point on the atrial septum. At this point, some operators like to move to the lateral fluoroscopic position to check that the needle is indeed pointing posteriorly. With the catheter held steady, the needle is advanced. If it crosses the atrial septum, a slight “give” is often felt or a slight movement seen. The catheter/needle is held completely static with the left hand until the entry of the needle into the LA cavity has been confirmed by the presence of at least two of the following: recording of an LA pressure trace, aspiration of bright red oxygenated blood, or contrast injected through the needle is seen to swirl within the LA cavity. The catheter can then be advanced, but is immediately rotated to a 3 o’clock position to avoid the posterior LA wall.
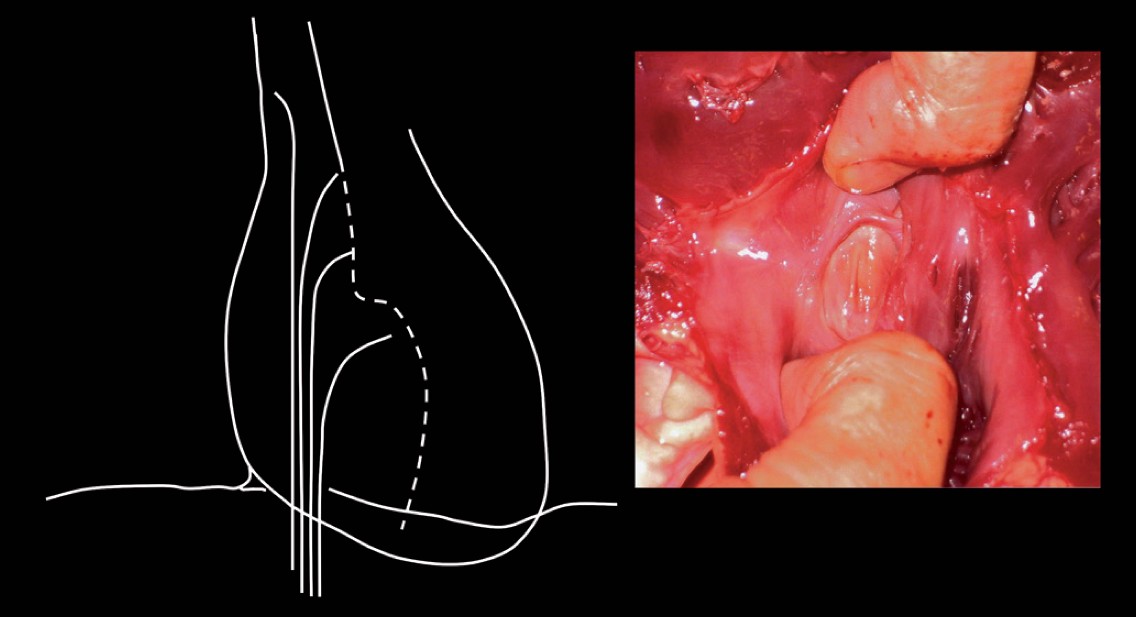
Finding the limbic edge. Left panel: schematic illustration of withdrawing the catheter needle assembly from the superior vena cava to the fossa ovalis. Right panel: pathological specimen showing fossa ovalis with the superior limbic edge.
This detection of the limbic edge is found in over 90% of structurally normal hearts and is the positioning method employed in the majority of electrophysiological cases. During the catheter/needle manipulation, many operators have the hub of the needle connected to a pressure monitoring line to allow immediate identification of the change from RA to LA. Many operators also routinely maintain a pigtail catheter in the right coronary sinus to help identify the position of the aorta. If the patient shows any tendency to coughing this should be advised against at the time of puncture.
When the secundum and primum septa are not fused, the catheter/needle may cross with no or minimal upward pressure.
When there is structural heart disease and the LA is dilated, this method frequently fails to detect the limbic edge. In this circumstance, the help of visualisation is required. The simplest form of visualisation is fluoroscopy. When the LA is enlarged it is usually visible within the cardiac silhouette and attempts can be made to find a catchpoint on upward pressure within the lower half of the LA silhouette.
Injection of contrast (e.g. 30 mL at 12 mL/s) into the RA with follow-through imaging to the LA allows the area of overlap of the two atrial cavities to be identified. In the anteroposterior position, this can be memorised in relation to the vertebral bodies. Many operators use this to identify the target area for puncture, particularly when there is distorted intracardiac anatomy (Figure 10).
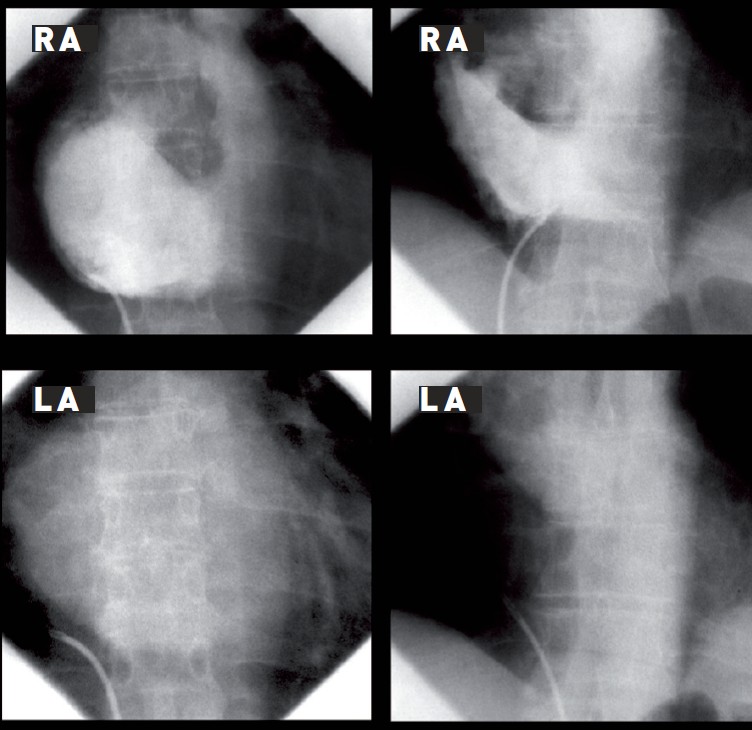
Right atrial contrast injection with follow-through to identify RA/LA overlap in anteroposterior projection. Left panel: extensive overlap in mitral stenosis. Right panel: marked anatomical variation in a patient with aortic stenosis and an intrapericardial cyst. Successful puncture was achieved. LA, left atrium/atrial; RA, right atrium/atrial.
A further step in visualisation to achieve safe puncture has been described by Inoue et al. and Hung . In Inoue et al.’s method, contrast is injected into the RA and followed through until the LA cavity has been visualised (Figure 11). A horizontal line is imagined from the superior end of the tricuspid valve to the right lateral edge of the LA. From the midpoint of this line, a vertical line is imagined down to the caudal edge of the LA. The recommended puncture site is on this vertical line at a distance from the caudal LA edge of just less than the height of one vertebral body. Hung’s method uses only fluoroscopy. A pigtail catheter is placed deep within the right coronary sinus; this corresponds closely to the upper end of the tricuspid valve. A horizontal line is imagined from this point to the right lateral wall of the LA fluoroscopic silhouette. The puncture point is then chosen as with Inoue et al.’s method (Figure 11). However, regardless of where one would like to cross visually, it is essential to find a catchpoint for the catheter/needle tip. The fossa ovalis, which is of oval or circular shape, usually with an area of 1.5−2.4 cm2, is composed mainly of thin fibrous tissue and is the easiest and safest part of the septum for a standard puncture. The rest of the septum is thicker and consists primarily of atrial muscle fibres but is also capable of being crossed. In valve disease, the position of the fossa ovalis may alter, and crossing may be successfully made outside the fossa . When the LA is enlarged, the fossa tends to be displaced inferiorly (Figure 12).
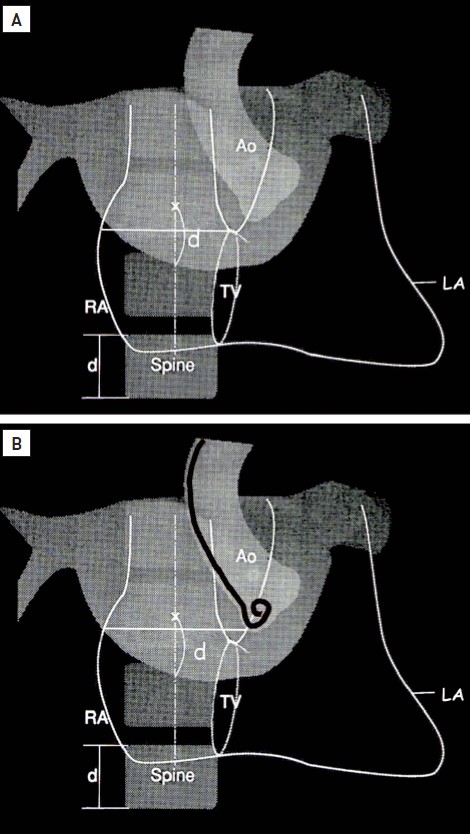
(A) Inoue et al.’s method, using right atrial to left atrial contrast. (B) Hung’s method, using fluoroscopy. Ao, aorta; d, distance; LA, left atrium; RA, right atrium; TV, tricuspid valve.
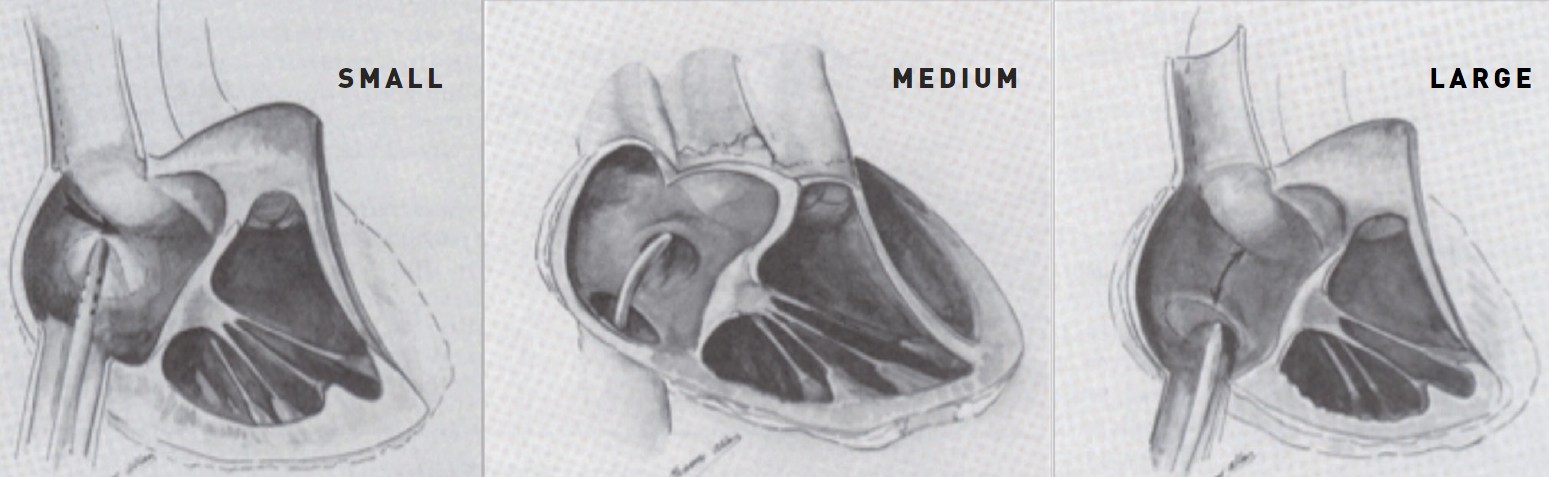
Transseptal puncture in small, medium, and large left atrium.
The steps for this fluoroscopic position are similar to those described above, but in the 40−50° right anterior oblique (RAO) view, the atria are superimposed and “en face”. The fossa is usually located 1−3 cm below the tip of a pigtail catheter in the aortic root and between the pigtail and the posterior RA silhouette.
Electrophysiologists also use the left anterior oblique (LAO) view. In this view, one is looking end-on to the atrial septum (i.e. the opposite of the en-face view seen in the RAO position). The electrophysiologists have the advantage of having a diagnostic electrophysiological catheter in the coronary sinus to mark the caudal end of the septum as well as a pigtail catheter in the aortic sinus or a His bundle catheter to mark the upper aspect. This view confirms when the needle curve is perpendicular to the atrial septum (see below).
In the lateral projection, the aortic root is first marked by a pigtail catheter deep in the aortic sinuses. The transseptal needle should then be oriented medially or slightly posterior to avoid puncturing the aorta anteriorly and the posterior wall of the RA posteriorly.
It is preferable to make the descent from the SVC in the anteroposterior projection. When descending with the catheter/needle, the operator should observe two jumps − one from the SVC to the RA and one from the RA to the fossa. On reaching the level of the aortic root marked by the pigtail catheter tip, it is advisable to move temporarily to another projection (e.g. RAO) to confirm the posterior orientation of the needle. An imaginary horizontal line is then drawn passing the aortic root to the spine. The puncture site would be 3−5 mm below that line (Figure 13).
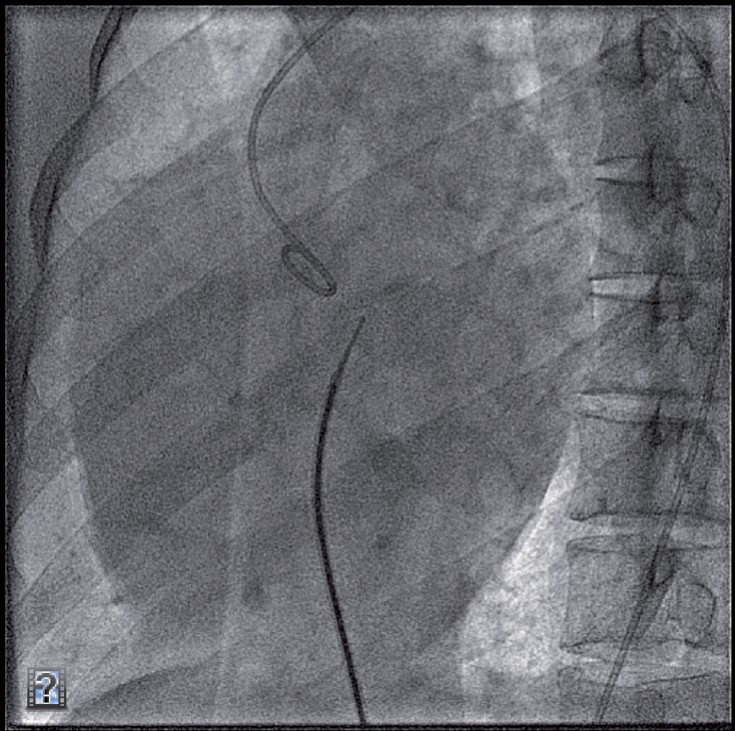
Lateral projection before transseptal puncture.
Routine diagnostic transseptal cardiac catheterisation per se in the hands of experienced operators does not need echocardiographic guidance in most cases. However, it is a tremendous imaging tool to help operators with less experience or if it is anticipated that there might be difficulty with transseptal puncture due to unusual anatomy or pathology of the atrial septum. It is also the only current technology that allows for a very specific site puncture, as is required in a myriad of interventional procedures such as LAA exclusion, edge-to-edge mitral valve repair long-tunnel PFO closure, closure of mitral paravalvular leaks, and pulmonary vein stenting (Figure 14) .
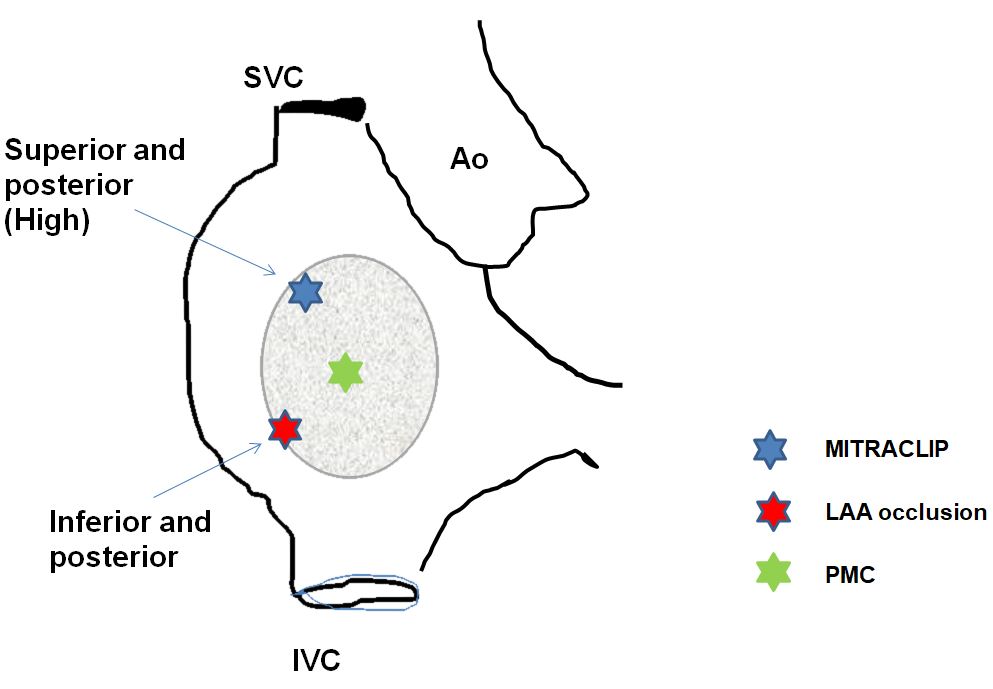
Specific site of transseptal puncture according to indication. Ao: aorta; IVC: inferior vena cava; SVC: superior vena cava.
It must be emphasised that the use of echocardiography in many forms can significantly improve visualisation of the atrial septum during puncture . Intracardiac echocardiography, TOE and occasionally TTE can all help to show the tenting of the septum before puncture. They can also help to determine the best portion of the atrial septum for puncture. The use of echocardiographic guidance enables selection of the site of the puncture within the fossa ovalis according to the expected type of procedure (e.g. anterior and posterior puncture for edge-to-edge mitral repair versus a lower and posterior puncture for LAA occlusion).
It is important in this case to use the following terminology to have a good communication between echocardiographist and interventional cardiologist. Superior: towards SVC, inferior: towards IVC; anterior: towards aorta, posterior: opposite from aorta; low: close to mitral valve; high: far from mitral valve (Figure 15).
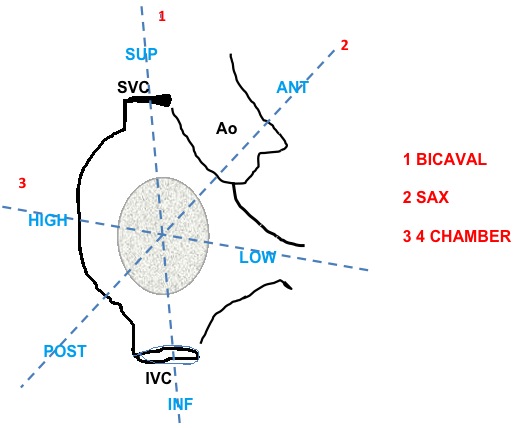
Echocardiographic terminology for site selection of transseptal puncture. ANT, anterior; Ao, aorta; INF, inferior; IVC, inferior vena cava; POST, posterior; SAX, Short Axis; SUP, superior; SVC, superior vena cava.
The safety of the echocardiographically guided transseptal puncture is supported by reports of success rates of 99% with few complications . If two LA catheters are used during an ablation, even a 1-cm difference in the location of the puncture site can make a difference in ease of manoeuvring a mapping and ablation catheter
Intracardiac echocardiography (ICE) is widely used in the United States, as demonstrated by the fact that approximately 50% of the experts involved in the 2018 consensus statement on AF ablation routinely used ICE to facilitate the transseptal procedure or guide catheter ablation . ICE does not require heavy sedation or general anaesthesia, as are commonly required in patients undergoing TOE. However, currently available ICE probes are monoplanar (with or without Doppler capabilities), while TOE is multiplanar and some also have three-dimensional capabilities. In addition, ICE catheters are expensive and have significant cost implication whereas TOE probes are reusable. TOE is generally thought to give better imaging during transseptal puncture and, most importantly, during the performance of the subsequent steps in structural interventions.
Alternatively, micro-TOE probes have been developed (micro TOE S8-3t Philips Healthcare Medical Systems, Andover, Massachusetts). The tip width is 7.5 mm, which is much better tolerated than conventional probes in conscious patients. It allows two-dimensional multiplanar, colour imaging with pulsed and continuous Doppler. Better tolerance allows to perform the procedure under conscious sedation. However, image quality as well as Doppler performance are lower than with a conventional probe.
If the transseptal puncture is guided by TOE (Figure 16) three two-dimensional TOE planes are sequentially used to precisely define the preferred puncture site: (1) long-axis view (bicaval) at 90−120°, which allows for superior−inferior orientation; (2) short-axis view at the base (30−50°), which allows for anterior−posterior orientation; (3) four-chamber view (0°), which is used to determine the correct height above the mitral valve, which is mandatory when performing the MitraClip and other percutaneous mitral procedures.
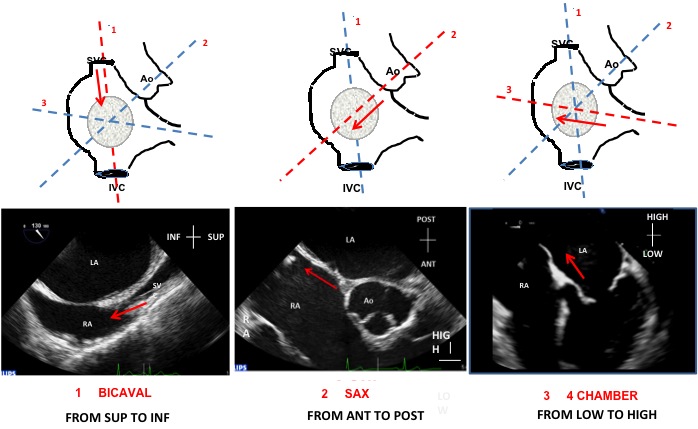
Use of the three sequential transoesophageal echocardiography planes (schematic illustration in upper panel, echocardiograms in lower panel) to guide transseptal puncture: (A) bicaval, from superior to inferior; (B) Short-axis view, from anterior to posterior; and (C) four chamber, from low to high. ANT, anterior; Ao, aorta; INF, inferior; IVC, inferior vena cava; LA, left atrium; POST, posterior; RA, right atrium; SUP, superior; SVC, superior vena cava.
The position of the transseptal puncture needle can be identified by a tent-like doming of the interatrial septum towards the LA (tenting) (Figure 17). Successful crossing of the septum is confirmed by the abrupt loss of tenting.
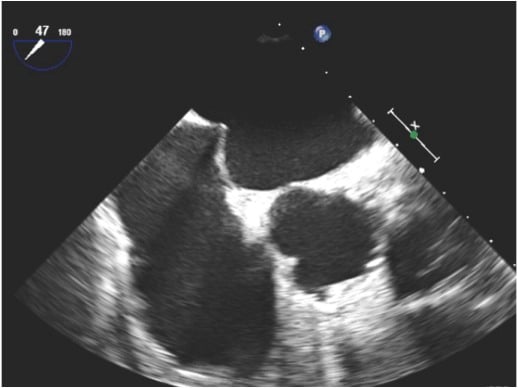
Identification of a tent-like doming of the interatrial septum (tenting) by transoesophageal echocardiography.
Three-dimensional X-plane imaging can facilitate the transseptal puncture by presenting short- and long-axis views simultaneously, thus providing antero−posterior and superior−inferior orientation in a single view.
When using ICE imaging, tenting of the interatrial septum should be identified in the long-axis view and clear visualisation of the point of maximal tenting should be obtained before puncturing (Figure 18). The relationship with the aortic root can be obtained by counter-clockwise rotation of the ICE catheter. Confirmation of the location of the ICE catheter in the LA can be obtained by injection of non-agitated saline or contrast during ICE imaging or the presence of a left-to-right shunt with colour Doppler.
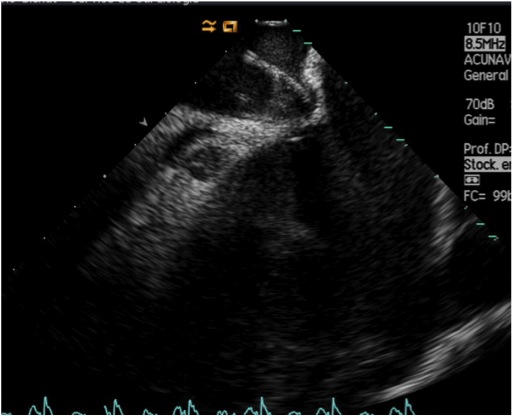
Identification of a tent-like doming of the interatrial septum by intracardiac echocardiography.
TTE guidance is seldom used because it is difficult to perform at the same time as fluoroscopic imaging. However, it could be helpful in experienced hands.
Isolated case reports have described the feasibility of transseptal puncture on echocardiographic guidance alone, without fluoroscopy, in emergency cases. However, this approach cannot be recommended presently.
Fusion imaging of fluoro and TTE is not routinely feasible, but might be helpful in selected cases .
Whatever the imaging technique used, it requires a good understanding and collaboration between the interventionist and echocardiographist, as well as the use of similar definitions, to optimise the performance of the procedure.
If the right femoral vein is not accessible, transseptal puncture can be achieved via the left femoral vein. Displacement of the veins may produce significant abdominal discomfort. Displacement may be reduced by moving the patient’s upper body towards the right. Some operators advocate that the left femoral vein could help to achieve a higher position of the delivery system for edge-to-edge repair .
If neither femoral vein can be used, if the IVC is occluded, or when there is azygous continuation of the IVC, it is possible to perform transseptal puncture via the SVC. Access is via the right internal jugular vein, and a paediatric Brockenbrough needle or an Endrys needle set is used to cross the atrial septum. If the atrial septum is not bulging towards the RA, extra curvature may need to be given to the needle. This approach has been used for mitral balloon valvuloplasty .
It is also possible to reach the RA via the hepatic veins. This alternative route is used exceptionally. It can be used when there is an interrupted IVC, either congenital – as in patients with atrial isomerism – or acquired (e.g. extrinsic pressure from tumours or intrinsic obstruction by thrombus, tumours, or devices). It can also be used when severe spinal deformities cause marked tortuosity of the IVC, when both femoral veins are chronically occluded, or when there is an absence of a right-sided SVC.
This technique should not be used if there is active peritonitis, an active coagulation disorder, and in some forms of anomalous hepatic vein drainage.
The thoracoabdominal skin area is surgically prepared and draped. The procedure is done under general anaesthesia and all procedures are guided by fluoroscopy, abdominal Doppler-ultrasound, and TOE. A 22 G Chiba needle is advanced under abdominal Doppler-ultrasound guidance into the hepatic vein. Saline injected through the needle identifies microbubbles passing into the RA. Additional contrast injection under fluoroscopy will confirm the location of the needle. A 0.018” guidewire is then advanced through the needle into the RA. The needle is then exchanged for a curved 9−10 Fr sheath. A 0.032−0.035” guidewire is then advanced into the SVC, and an 8 Fr Mullins sheath is advanced through the larger transhepatic sheath. The Brockenbrough needle is then advanced and a descent from the SVC into the interatrial septum is performed, as previously described for the femoral approach. Septum puncture is guided by TOE. After completing the transseptal cardiac catheterisation/intervention and anticoagulation has been reversed, the transhepatic sheath is slowly withdrawn while a diluted contrast injection through the side arm identifies, by fluoroscopy, the exit of the sheath from the hepatic vein into the hepatic parenchyma. Coils or an Amplatzer® Vascular Plug (St. Jude Medical, S. Paul, Minneapolis) is then used to seal the hepatic vein access.
There are several situations in which the nature of the atrial septum can add technical difficulty to achieving successful transseptal puncture.
The septum may prove resistant to needle puncture even at the fossa ovalis. This tough, or resistant, septum is more likely to be found if there have been several previous transseptal punctures or previous cardiac surgery , in older patients, or after thoracic radiation.
In case of a resistant septum, one should keep in mind that the puncture site might be in the muscular part of the septum rather than in the fossa, and that it may be necessary to redo the puncture at another site.
If in a good position, increasingly forceful forward pressure on the needle may be sufficient to overcome the resistance. While this is usually safe with an enlarged LA, care is needed with a normal-sized LA.
A resistant atrial septum can pose a hazard because of the extra forward pressure needed to complete atrial puncture. Even if the needle alone penetrates the septum safely, passage of the Mullins dilator and sheath over the needle may also necessitate unusual forward force. This can result in a sudden forward acceleration of the needle tip/dilator/sheath combination as the atrial septum is finally bridged. The stored energy from this forward pressure can then propel the needle, dilator, and sheath quickly across the LA before the operator can respond by pulling back, and LA perforation can occur. A simple trick to avoid a complication is to advance the needle alone until it just penetrates the LA. A change in the pressure recording from the needle tip indicates passage into the LA.
Use of radiofrequency usually helps to overcome resistant septum and the Baylis system is increasingly used on a routine basis. By using the Baylis catheter in such circumstances, excess forward pressure, which could result in unsuccessful puncture or perforation of the LA free wall secondary to catheter momentum after puncturing the septum, is avoided .
Radiofrequency energy can also be used in a simple way via the Brockenbrough needle by using a standard electrosurgical cautery generator. Brief pulses of radiofrequency energy are applied to the hub of the transseptal needle either by direct contact or via a double crocodile clip connector wire. This is a very effective way of overcoming a resistant septum without the use of excessive force, which improves the safety of the procedure and also eases the performance of the transseptal puncture at a desired site for structural interventions.
It should be stressed that these additional techniques should only be used when the location of the transseptal catheter is precisely shown by “tenting”, imaged by ICE or TOE.
If the needle achieves a catchpoint but does not enter the LA cavity, some operators elect to inject a very small volume of contrast through the needle. It is said that if there is a vertically aligned stain of contrast then the position is too high, and if horizontally aligned the position is too low.
If the needle can cross, but not the catheter, it may be useful to introduce an exchange angioplasty 0.014 guidewire into the needle up to the left superior pulmonary vein and use it as support for both the needle and the catheter. As an alternative to this technique, the 0.014” angioplasty guidewire can instead be looped into the LA. Passage of the sheath and dilator will then be over the looped guidewire, avoiding rapid transit to the opposite side of the LA .
After transseptal puncture, the device (e.g. Inoue balloon, MitraClip) might not cross the septum. In this case, the puncture point can be enlarged by dilating the crossing point with a balloon (8−14 mm according to device size).
If asked, patients will occasionally report a mild feeling of discomfort when puncture occurs. Very occasionally, however, a patient will immediately declare major discomfort with even modest pressure on the septum. This tender septum can usually be dealt with by use of intravenous opiate, but, rarely, the tenderness is so exquisite that general anaesthesia is required.
When LA pressure is very high, as it may be with severe mitral stenosis, there may be a markedly bulging septum towards the RA cavity. As a result, when the catheter/needle descends from the SVC it can repeatedly slip medially or laterally because of the bulge. Forceful torquing of the needle back onto the middle of the septum bulge usually allows successful entry to the LA.
A giant LA might be thought to make transseptal puncture easier, but the opposite is often the case. It can be very difficult to find a catchpoint on the septum by the catheter or needle tip. Often, an unusually low, avoiding too posterior, position on the septum has to be used (Figure 12).
It should be stressed that a too-posterior puncture, outside the borders of the fossa ovalis, should not be used to avoid a perforation into the pericardial space between the two atria. In such cases, the convexity of the septum due to LA enlargement may cause catheter slippage and may thus impede adequate contact with the septum.
A very large RA can make it difficult for the needle curve to reach the septum. In such cases, it is useful to slightly bend the needle at 10 cm from the tip to facilitate contact with the septum (Figure 19) or to use a needle with a more pronounced curve (e.g. BRK1 instead of BRK).
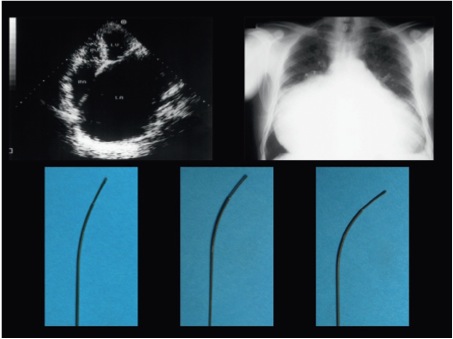
Very marked enlargement of the left atrium (left) on echocardiography and right atrium (right) on X-ray with the needle curve (lower panel) altered to adjust to the abnormal anatomy.
An atrial septal aneurysm directed into the RA cavity may be seen on preprocedural TTE. Crossing may be surprisingly easy as there is a higher incidence of associated PFO . (Figure 20). However, if there is resistance, the operator has to be cautious not to puncture the posterior LA wall when pressure causes the aneurysm to prolapse into the LA. When marked tenting of the septum is suspected, the posterior LA wall can be protected using a sharp Nitinol J-tipped SafeSept™ wire or by advancing an angioplasty wire through the needle .
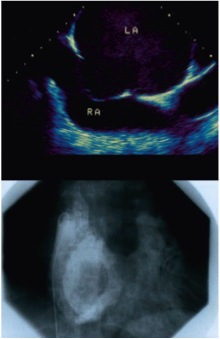
Atrial septal aneurysm as seen by echocardiography (upper panel) and on contrast injection into the LA (lower panel). LA, left atrium; RA, right atrium.
In general, when faced with a difficult septum, several passes down the septum may be required. In this case, the VersaCross (Baylis Medical, Montreal, Canada) allows the system to be safely readvanced towards the superior vena cava by pushing the wire to its pigtail shape. As an alternative to repeated exchanges between needle and guidewire to regain access to the SVC, the “anterior staircase” manoeuvre can be used (Figure 21). When the catheter/needle reaches the inferior portion of the LA, it is rotated to have the curve pointing to 12 o’clock. The catheter/needle is then advanced to the top of the LA while moving the tip continually from side to side to confirm no snagging. As the IVC enters the RA posteriorly there is space within the RA cavity to accommodate the curve. The catheter/needle is then rotated again to a posterior position and drawn down the septum.
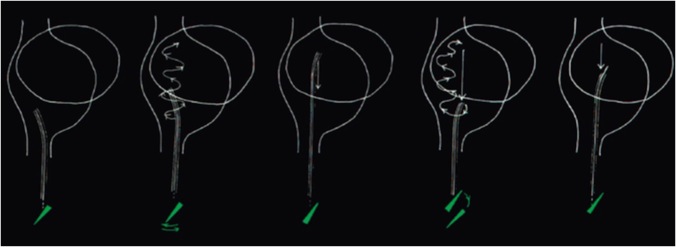
The "anterior staircase" technique. When the catheter/needle reaches the inferior portion of the LA, it is rotated to have the curve pointing to 12 o’clock. The catheter/needle is then advanced to the top of the LA while moving the tip continually from side to side to confirm no snagging. As the inferior vena cava enters the RA posteriorly there is space within the RA cavity to accommodate the curve. The catheter/needle is then rotated again to a posterior position and drawn down the septum. LA, left atrium; RA, right atrium.
If achieving successful puncture is causing delay, the needle should be withdrawn every 4−6 minutes and the catheter back-flushed to remove any thrombus that develop on the needle.
For children, the most common indication for a transseptal puncture is the balloon mitral valvuloplasty procedure, particularly in developing countries . The septum is usually easy to cross but in some cases a fibrous and thick septum can be encountered. In such cases, care should be taken to avoid a jump of the needle from the RA to the LA, which can cause damage to the atrial wall resulting in tamponade.
Any procedure involving ionising radiation should be avoided during the first trimester whenever possible. Transseptal puncture should be performed in the usual way using the normal landmarks. The catheter/needle usually passes easily under the gravid uterus. However, to minimise the risk of foetal radiation, the patient’s abdomen should be shielded by a lead apron placed between the patient and the direction of the X-ray beam. For the same reason, the use of fluoroscopy should be minimised and cineangiography avoided. However, patient safety should never be compromised by excessive minimisation of the technique and, if available, general anaesthesia is useful in this setting to allow for TOE guidance. Transseptal catheterisation is performed during pregnancy mainly for a percutaneous balloon mitral valvuloplasty procedure. The transseptal puncture and balloon dilatation do not need much fluoroscopy time and studies have shown no deleterious radiation side-effects in the babies after these procedures . In some cases, the patient needs to be in a 45° lateral position to prevent the pressure of the uterus reducing IVC flow and aggravating hypotension. This non-standard position needs to be taken into account when controlling the angulation of the needle; however, such a position should be avoided if possible.
Dextrocardia is a rare condition found in about 1 in 12,000 of the general population. Almost one-third of these persons have situs inversus . When dextrocardia is not associated with situs inversus, the heart is just located in the right side of the thorax. In that case, the transseptal puncture is performed as in the normal cases with the same SVC descent and intracardiac landmarks. However, when it is associated with situs inversus, the major visceral organs including the heart are mirrored from their normal positions. The transseptal puncture is, in that case, associated with marked technical difficulties and a high risk of cardiac perforation. There are two suggested ways to deal with the transseptal puncture in this setting, either to invert the image of the fluoroscopy screen but keep thinking you are manipulating in the opposite direction as usual, or keep the image as it is but concentrate with the help of a cooperator. It may be preferable to use the left femoral vein (Figure 22). Again, TEE guidance is helpful in this situation.
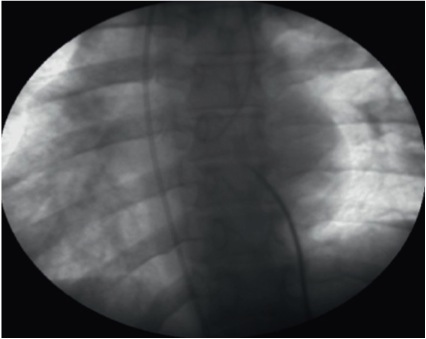
Transseptal puncture in case of dextrocardia situs inversus.
Interventional electrophysiologists have used transseptal puncture since the 1980s as an alternative or complementary approach to the retroaortic route to ablate left-sided accessory pathways. However, the spread of the technique in the electrophysiological laboratory has followed the development of LA ablation to treat AF.
The techniques of transseptal puncture have similarities, whether used in the electrophysiological laboratory or in the haemodynamic or structural intervention laboratory, but some specific features deserve to be mentioned.
The main procedure categories are:
In addition, transseptal catheterisation is occasionally used to enable endocardial placement of a left ventricular pacing lead for patients requiring cardiac resynchronisation therapy in whom left ventricular lead placement cannot be satisfactorily achieved via the coronary sinus.
Specific features of transseptal puncture in the electrophysiological laboratory include the double transseptal technique. In most cases, more than one catheter is used for LA ablation. This is done using either a double transseptal puncture or a single puncture. In the former, a guidewire is placed in the left superior pulmonary vein through the first sheath and dilator, which are then pulled back into the RA. Next, the ablation catheter (through a second sheath) is positioned (under fluoroscopy and/or TEE or ICE) in the fossa ovalis and pushed into the LA following the guidewire direction. Finally, the first sheath and dilator are pushed over the wire into the LA. An alternative method for the double transseptal technique with a single puncture has also been described .
Placement of diagnostic electrophysiological catheters before transseptal puncture can provide helpful anatomical information to the operator. A quadripolar catheter placed in a position where a His bundle electrogram is recorded will identify the site of the inferior aspect of the non-coronary aortic cusp. A multipolar catheter placed in the coronary sinus will mark the orientation of the atrioventricular groove, and together, these catheters provide the operator with an index of cardiac rotation. A LAO fluoroscopic view can be adjusted so that the bundle catheter is viewed end-on (Figure 23). This will approximate the view along the axis of the interatrial septum. The transseptal sheath and needle can then be correctly orientated perpendicular to the septum before puncture. This view is also helpful in mapping left-sided accessory pathways, in which cases the coronary sinus catheter circumscribes the posterior and lateral mitral annulus.
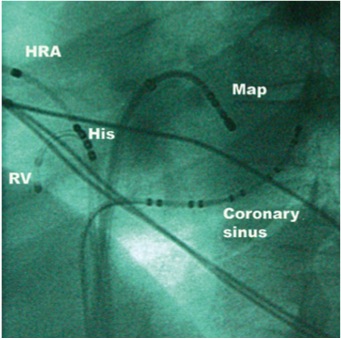
Left anterior oblique fluoroscopic view of electrophysiology catheters. HRA, high right atrium; RV, right ventricle.
Approximately 70% of accessory pathways have a left-sided location and span the mitral annulus.
Once a left-sided pathway has been identified, two approaches may be used. The ablation catheter can be guided to the mitral annulus retrogradely via the arterial circulation from the femoral artery, or it can be delivered to the mitral annulus via a transseptal sheath. The retrograde approach can be technically challenging because of the difficulty in steering the catheter as it traverses the aortic arch and is retroflexed towards the mitral annulus. Ablation catheters are quite rigid in build and have the potential to plug or injure a coronary artery, or to cause cerebral embolism. Catheter placement can also be limited by peripheral vascular disease or aortic aneurysm, although these pathologies are uncommon in most affected patients who present in early adulthood with their arrhythmias.
The transseptal approach is advantageous for ablation of left-sided accessory pathways because the catheter is naturally directed toward the lateral mitral annulus after passage through the intra-atrial septum. Puncture is normally straightforward because most patients have no associated structural heart disease and therefore normal anatomy. A Mullins transseptal sheath can be used for the initial puncture, but better support is achieved if a dedicated support sheath is used. Preshaped sheaths are available that allow access to different positions on the mitral annulus, providing catheter stability, which is essential to deliver an effective lesion to the accessory pathway. Left posteroseptal and paraseptal pathways are the most difficult to access as the catheter has to be delivered anterior to the puncture site. This is achieved either by firm anticlockwise rotation of a short-nosed transseptal sheath or by looping the catheter all the way around the mitral annulus, where a dedicated support sheath is often needed for support.
Atrial tachycardias are usually focal in origin, but can be macroreentrant (i.e. LA flutter). LA tachycardia may originate from any site in that chamber, but the antra of the pulmonary veins, the LAA, and the mitral annulus are the most common sites. Pulmonary vein foci are also the most common initiators and drivers for AF. Pulmonary vein isolation techniques are used, either alone or in combination with linear ablation and focal LA ablation, to prevent AF. The two most used approaches are segmental pulmonary vein isolation and circumferential ablation, where the left- and right-sided veins are encircled by a continuous ablation lesion set to achieve isolation.
Pulmonary vein anatomy is highly variable between individual patients in terms of vein size, orientation, and number. There is corresponding variability in difficulty achieving proper contact between catheter and vein antrum during the ablation procedure. LA ablation procedures may involve placement of two catheters into the left heart – one reference catheter and one ablation catheter. During ablation for AF, a circular multielectrode catheter is normally placed in the pulmonary vein ostium to allow identification of conducting tissue and to verify vein isolation during ablation. A double transseptal puncture technique is normally employed, using two separate but adjacent punctures. While it is possible to pass two transseptal sheaths through a single puncture hole using a double guidewire exchange technique, sheath manipulation is often constrained by immediate proximity of the adjacent sheath. Fixed curve sheaths for LA ablation have a leftward pointing tip to direct the catheter towards the posterior LA, where the pulmonary veins are situated, whereas the sheaths used for accessory pathway ablation tend to direct the catheter anteriorly towards the mitral annulus. For patients with difficult anatomies, steerable transseptal sheaths may be used (e.g. St. Jude Agilis, St.Paul, Minnesota and Bard Channel, Bard Inc., Murray Hill, New Jersey). These sheaths can be flexed and extended to provide an additional degree of freedom for catheter placement, but have the disadvantage of the larger outer diameter (11.4−13 Fr) required to accommodate the deflection mechanism.
Monomorphic ventricular tachycardia (VT) can occur through several mechanisms. So-called “normal heart” VT can arise from the left ventricle as verapamil-sensitive fascicular VT, and from the left ventricular outflow tract. Bundle branch reentrant VT and interfascicular VT usually occur in association with non-ischaemic dilated cardiomyopathy. All of these forms of VT are amenable to ablation using a retrograde aortic approach because septal or outflow tract tissue is readily accessible using that approach. However, in patients with VT occurring due to coronary artery disease, left ventricular scar normally forms the substrate. This can occur at any site in the left ventricle. Although not the approach of choice, transseptal catheterisation may be helpful in some cases where catheter manipulation is constrained using the retrograde approach .
See chapter Atrial septal defect and patent foramen ovale closure
The presence of a long-tunnel PFO poses a challenge for many of the currently used devices. An alternative to transtunnel device placement has been described , consisting of selective transseptal puncture of the most cephalad aspect of the septum primum at the fossa level in order to place a device that can sandwich both septum primum and secundum and effectively closing the PFO tunnel without causing major deformity or misalignment of the devices.
Transseptal catheterization can also be used during percutaneous left ventricular assist device implantation.
The Tandem Heart (Cardiac Assist Technologies Inc., Pittsburgh, Pennsylvania) is a transseptal LA-to-femoral arterial assist device that provides left heart bypass designed for short-term circulatory support. The inflow transseptal cannula is a 21-Fr polyurethane catheter with a large end-hole and 14 side-holes to facilitate LA decompression.
Transseptal catheterization is also used in patients treated with venoarterial extracorporeal membrane oxygenation with persistent pulmonary oedema. In this situation, a venting canula is placed in the LA via transseptal catheterization and connected in the extracorporeal membrane oxygenation circuit using a Y connection .
The techniques to perform transseptal catheterisation safely in complex congenital heart disease vary considerably and depend on the structural anatomy of the underlying cardiac anomaly. The technique to enter the systemic atrium when there is drainage of the IVC into the venous atrium will very much depend on whether there is atrial (± visceral) situs solitus, inversus, or ambiguous. The needle/sheath descent from the SVC into the venous atrium will also depend on whether this vessel is in alignment with the IVC. Thus, transseptal puncture in complex congenital malformations requires a good understanding of the pertinent anatomy in three dimensions to direct the tip of the needle towards the correct orientation. When in doubt, of course, the position of the needle when tenting the interatrial septum can be visualised by echocardiography, before actually advancing the needle, to grant a higher safety margin. In the presence of congenitally absent IVC and azygous continuation to the SVC, as is seen in patients with atrial isomerisms, one can opt for a transhepatic−transseptal approach, if the hepatic venous drainage into the venous atrium is anatomically favourable, or attempt a transseptal puncture from an SVC approach.
See chapter Transcatheter mitral valve repair
The most common mitral valve repair technique used today is the edge-to-edge technique using the MitraClip. The transseptal puncture is performed under echo guidance, which is subsequently necessary for the performance of the procedure . The determination of the optimal puncture site is of major importance for a MitraClip procedure. The puncture must be performed in the superior−posterior part of the fossa ovalis and at an adequate distance from the mitral valve (Figure 14). The optimal height above the mitral valve differs for primary and secondary mitral regurgitation. In cases with primary mitral regurgitation, the puncture site should be 4−5 cm above the mitral annulus, thus providing enough space to adequately manoeuvre the delivery system within the LA. In patients with secondary mitral regurgitation, important valve tethering most often results in a shift in the position of the line of coaptation to below the mitral annulus. Subsequently, transseptal puncture needs to be lower, approximately 3.5 cm above the annular plane or 4−4.5 cm above the coaptation of both leaflets to be able to advance the catheter more deeply into the LA.
The bicaval TOE view allows superior or inferior orientation in the septum, while the short-axis view best shows how far “posterior” the puncture is to be made. Lastly, the four-chamber view shows the distance from the puncture site to the point of mitral valve coaptation (Figure 16). An ideal puncture should be made so that the distance to the line of coaptation in the four-chamber view is 3.5−4 cm to allow adequate space for clip manipulation and proper deployment in the mitral leaflets.
An inadequate puncture site will lead to the following complications: aortic hugging (if the puncture is too anterior, e.g. through a PFO); inability to cross the mitral valve in a too-high puncture; inability to pull back the clip and tether the leaflets after a too-low puncture; and risk of perforation if the puncture is too posterior.
The stability of the position of the needle should be carefully checked before pushing the sheath over the needle. For this purpose, the use of radiofrequency energy could be a useful adjunct by avoiding the potential slippage of the needle while applying force to cross the septum.
Experience of other mitral interventions is growing (e.g. “valve-in-a-valve” (or “valve-in-a-ring” or direct annuloplasty) , , . There is, however, no specific recommendation concerning the transseptal technique in such cases. As a general rule, the transseptal puncture for these procedures shares most of the characteristics of that used for the MitraClip technique (i.e. a superior and posterior puncture to allow for sufficient manoeuvrability in the LA and crossing of the mitral valve) , .
Paravalvular leak (PVL) occurs in 5−17% of patients undergoing surgical valve replacement and may have clinical consequences such as congestive heart failure or haemolysis. Percutaneous closure of PVLs is currently the first-line treatment of symptomatic PVL. Some operators recommend adapting the transseptal puncture according to the location of the PVL . In this case, a high transseptal puncture is preferred for a lateral PVL (6−12 o’clock) and a lower puncture is preferred for a septal PVL (12−6 o’clock). This point is, however, a matter of debate since other groups recommend a high transseptal puncture regardless of the location of the PVL.
See chapter Left atrial appendage occlusion
The LAA is variable in its size, shape, and position. Its orifice usually lies almost directly across the LA from the site of the fossa ovalis, though variability in the size and exact position of the orifice is common.
Here again, the location of the transseptal puncture is important and somewhat dependent on LAA anatomy to facilitate coaxial alignment of the device and reduce the risk of complications such as tamponade or embolization. Considering the anatomy of the LAA (superior with an anterior orientation in most cases), the ideal location for transseptal puncture is most often inferior and posterior, which allows a coaxial engagement of the appendage (Figure 14). This has, however, to be adapted depending on patient anatomy .
Slight counter-clockwise rotation of the delivery sheath may be required to reach an LAA orifice that is positioned anteriorly.
See chapter Percutaneous balloon mitral commissurotomy
The optimal position for transseptal puncture for balloon mitral valvotomy is either directly in the centre of the fossa ovalis or, preferably, slightly “low” in the fossa (Figure 14). Such a puncture site allows the transseptal sheath and, ultimately, the balloon dilation catheter to follow a trajectory slightly above and across the plane of the mitral annulus. This allows the balloon dilation catheter to engage and cross the stenotic mitral valve with only a minimal bend toward the apex of the left ventricle. A “low” puncture is particularly important if the rarely used double-balloon technique is employed, though even with the Inoue balloon catheter, it facilitates crossing of the mitral valve. However, if crossing the mitral valve is not possible using this puncture location, it may be necessary to redo the transseptal puncture in a slightly higher position and more posteriorly .
The optimal position for transseptal puncture for balloon mitral valvotomy is either directly in the centre of the fossa ovalis or, preferably, slightly “low” in the fossa (Figure 14). Such a puncture site allows the transseptal sheath and, ultimately, the balloon dilation catheter to follow a trajectory slightly above and across the plane of the mitral annulus. This allows the balloon dilation catheter to engage and cross the stenotic mitral valve with only a minimal bend toward the apex of the left ventricle. A “low” puncture is particularly important if the rarely used double-balloon technique is employed, though even with the Inoue balloon catheter, it facilitates crossing of the mitral valve. However, if crossing the mitral valve is not possible using this puncture location, it may be necessary to redo the transseptal puncture in a slightly higher position and more posteriorly .
The presence of the guidewire and catheter/needle in the RA or LA can trigger an atrial tachyarrhythmia. These are usually self-terminating, but occasionally intravenous antiarrhythmic therapy is needed.
As with any cardiac catheterisation, the patient may become vasovagal at any stage and require drug or fluid therapy. There are several case reports of a much more intense and treatment-resistant bradycardia/hypotension. This response has been ascribed to a Bezold-Jarisch-like reflex triggered by irritation of fibres within the atrial septum . It is sometimes accompanied by transient ST elevation in the inferior leads: immediate coronary angiography has shown no abnormality and it has been postulated that this reflex has triggered coronary spasm although it is difficult to exclude the possibility of micro air-bubbles .
A very intense bradycardia and hypotension can also rarely be caused by bruising within the mediastinum after an apparently successful septal crossing. In this scenario, the catheter may have crossed outside the atria by traversing a cleft between the two enlarged atria. If this situation is suspected, it is vital not to pull back the catheter, but rather to send the patient to surgery. Immediate removal of the catheter can cause the patient to exsanguinate on the catheterisation table.
Meticulous care is required to avoid entry of air into the catheter or needle. Regular back-flushing of the catheter is needed if the puncture procedure is prolonged, as the stainless steel needle can induce thrombosis. The use of TOE/ICE has shown that instrumentation of the atria can cause small thrombi within the cavities. In patients undergoing LA ablation for AF, ICE identified thrombi in 10.3%: the thrombi were attached to a sheath or mapping catheter and most were drawn back to the RA when the catheters were withdrawn .
If, after advancement of the catheter/needle, there is no recording of LA pressure, the atrial wall may have been perforated. The risk of perforation is higher if the septum is resistant or the LA cavity small. If only the needle has perforated, it can be withdrawn safely (a situation similar to the early pretransseptal techniques), but if the much larger catheter has perforated, then major bleeding is almost inevitable, and the patient should be sent for catheter removal at open thoracic surgery. To differentiate those two circumstances, a tiny amount (0.1−0.3 mL) of contrast can be injected through the needle. If this causes staining outside the atrial cavity, then needle perforation is confirmed. The needle can then be withdrawn to just within the catheter tip while the catheter remains static. A further injection of contrast is given via the needle. If contrast swirls within the atrial cavity, the catheter has not exited the heart and can be withdrawn. If further staining is seen, the patient requires surgery for safe catheter removal .
If the patient is considered entirely unsuitable for surgery, there is an anecdotal report of effective catheter removal by passing a guidewire to the aorta and progressively downsizing the catheter size under echosurveillance. Percutaneous repair of aortic puncture with an Amplatzer closure device has also been described .
Advancement of the catheter/needle to outside the atrial cavities can cause bleeding into the pericardium. The first clinical signs are a drop in blood pressure and a diminution of movement of the cardiac silhouette. If the haemopericardium remains small or moderate and the clinical signs are stable, then the patient can simply be closely monitored. If cardiac tamponade is threatening, then immediate echo-guided aspiration is needed: blood can be autotransfused back to the patient. If the situation does not stabilise, referral for emergency thoracic surgery is required. In the early experience of transseptal puncture, cardiac tamponade occurred in 21 of 1765 (1.2%) patients . Eighteen responded to medical measures while two required surgery; two of the patients died.
The potential dramatic consequences of this complication require an immediate access to TTE when transseptal puncture is performed.
TOE or ICE has shown that there is almost always a small left-to-right shunt visible after any septal puncture procedure. However, follow-up echo studies show that only 15% persist after 6 months, and persistence is strongly correlated with the size of transseptal sheath. Current literature is inconclusive with regard to the clinical significance of persistent interatrial septal defect, but suggests a possible detrimental effect in some patients . Defect closure is not frequently performed and is based on integration of various parameters such as anatomy (large defect), characteristics of shunt (right to left), and clinical impact (shunting with hypoxia, volume overload).
Transseptal cardiac catheterisation is a technique that was routinely taught by many cardiology training programmes 30 years ago, but its use markedly declined as the need for haemodynamic information was being provided by alternative technologies with greater patient comfort and safety margins. The initiation of interventional procedures in the left cardiac chambers pioneered by balloon mitral valvuloplasty in the mid-1980s resurrected the need for transseptal catheterisation. However, the presence of a learning curve has been well described . Thus, there is a need for training courses that can teach the procedure both from the didactic and practical points of view. Such courses have recently been introduced to cater for the recently developed interventions (e.g. edge-to-edge mitral repair, LAA closure). The didactic lectures should include indications, contraindications, imaging, and step-by-step techniques in the presence of normal anatomy, as well as altered chamber size and different pathologies, and how to avoid, recognise, and resolve complications. Practical teaching may be obtained by performing a reasonable number of procedures with an experienced proctor. Today, however, there are simulators with excellent imaging information to teach the procedure step-by-step and some even have reliable tactile feedback .
Transseptal puncture into the LA was a clever solution to the problem of access to the heart’s most inaccessible chamber. Its use in accurately measuring the haemodynamics of left heart valve disease improved diagnosis and the selection of patients for valve replacement. However, the technique almost vanished with the emergence of two-dimensional colour flow Doppler echocardiography, and became virtually confined to centres undertaking transcatheter mitral valvuloplasty and some ablation procedures. During the past two decades, the use of transseptal puncture has risen massively due to two techniques – left heart ablation for AF, and then, since the turn of the century, mitral interventions and LAA occlusion. This has brought refreshing new ideas and devices to the technique, including radiofrequency energy to help cross a resistant septum and new imaging modalities. However, the biggest change has been the link-up between transseptal puncture and intra-procedure echocardiography – TOE or ICE. This also gives reassurance to inexperienced operators and to anyone dealing with very difficult distorted anatomy. With the new interventions, the search for a safe crossing point at the fossa ovalis has been replaced by a need to find a safe but site-specific crossing appropriate for the apparatus. Intra-procedure echo provides this. We believe the indications for transseptal puncture will continue to expand in the future and it will be used for transcatheter mitral valve implantation. It has been a rollercoaster ride for this old technique, but it has returned to mainstream cardiology. Learning side-by-side with a mentor will continue to be crucial, but the new training courses and simulators will help.
Horacio Medina de Chazal, Ali Zgheib, Angelo Quagliana, Michael Chetrit, Jean Buithieu, Giuseppe Martucci, Marco Spaziano, Ali Abualsaud, Ole de Baker, Laurence Campens, Pascal Theriault-Lauzier, Jere...
Updated on November 23, 2022
Mirjam Wild, Maurizio Taramasso, Alison Duncan, Giulio Russo, Dominique Himbert, Francesco Maisano, Nicolo Piazza, Fabien Praz
Updated on November 21, 2021
Olaf W. Franzen, Ole De Backer, Mirjam Wild, Lars Sondergaard
Updated on May 13, 2021
Mirjam Wild, Maurizio Taramasso, Alison Duncan, Giulio Russo, Dominique Himbert, Francesco Maisano, Nicolo Piazza, Fabien Praz
Updated on May 11, 2023