Thrombectomy and target vessel protection during PCI
Background
Prevention and management of coronary embolism is one of the greatest challenges during percutaneous coronary interventions (PCI), aiming at preserving microvascular integrity to warrant successful myocardial reperfusion.
Embolization can occur both in the context of Acute Coronary Syndromes (ACS), where large thrombus burden carries a twofold increase in mortality and a two-to-fourfold risk of major cardiovascular events, and during interventions on stable Coronary Artery Disease (CAD), where it's usually due to plaque iatrogenic fragmentation. Whatever the underlying mechanism and the interventional context, embolization has been shown to impair clinical outcomes by affecting microvascular patency.
Strategies to prevent these consequences have been theorized in the last decades. Thrombus debulking before conventional PCI in ACS achieved controversial results in clinical trials. As a consequence, aspiration thrombectomy in myocardial infarction has been downgraded in recommendations provided by European and American guidelines. By contrast, in the context of saphenous vein graft PCI protection devices have granted favorable results and are nowadays strongly supported.
In this chapter we review some of the pathophysiological mechanisms relating embolic phenomena to clinical outcomes and provide an overview on currently available thrombectomy and protection systems, with a glance on future perspectives for the prevention and management of embolic microvascular obstruction (MVO).
Sources and consequences of coronary embolism: a pathophisiogical insight
Incidence, sources and implications of embolic events
Coronary embolization can be a spontaneous event or a iatrogenic complication of percutaneous coronary interventions , . Although recognized much earlier as possibly related to sudden death in patients with unstable angina , , , , embolic phenomena have been addressed only in the last decades to find preventive strategies, devices and techniques. As invisible microscopic particles are frequently involved, the real overall incidence of embolic events is still difficult to assess.
Peri-procedural coronary microembolizations have been described to occur in about 25% of all PCIs, but their incidence ranges from 0% to 70% according to the assessment method , the clinical context and lesions’ and procedures’ complexity. Indeed, balloon angioplasty (i.e. number and duration of inflations), advanced lesion preparation techniques with atherectomy or rotablation, stent deployment and eventual post-dilatation are all possibly involved as potential triggers of embolic dislodgement .
Besides, in patients with acute myocardial infarction (AMI), the angiographic evidence of a distal filling defect due to macroscopic embolic phenomena - especially in patients with high thrombus burden - occurs in 7-16% of cases and is related to a reduced success of reperfusion, higher myocardial damage and a fivefold increase of 5-year mortality , , , .
In such a wide span of clinical scenarios, micro- or macroscopic debris´ migration leads or contributes to microvascular obstruction, which invariably carries relevant prognostic implications , , , , , , , .
Vulnerable coronary plaques, whose necrotic core is covered by a thin fibrous cap that is prone to erosion and rupture, are the most common source of embolic fragments , , , . Bioactive plaque content (i.e. foam cells, cholesterol) is higher in quantity in saphenous vein graft disease , , which explains a higher incidence of embolic complications during PCI in this specific anatomical context.
Acute coronary syndromes represent a more complex hystopathological scenario, where intraluminal thrombotic content is the leading source of embolic material. Restoring blood flow by wiring and ballooning the culprit lesion can itself be the trigger for thrombus migration, contributing to MVO and potentially leading to unsuccessful coronary reperfusion. Such hypothesis is nowadays universally accepted, supported by a huge bulk of evidence. Yip et al described how the incidence of no-reflow after PCI - as a marker of MVO - is significantly higher in patients with baseline angiographic signs of a large thrombus burden (LTB) .
Beside thrombus, intravascular imaging has provided additional insights in the relationship between the characteristics of the culprit plaque and the occurrence of no-reflow phenomena in ACS . Plaque volume reduction after stenting has been quantitatively related to fragments’ embolization and has been reported to be much higher (nine-folds) in patients with final TIMI flow 0-2 in comparison to patients with final angiographic evidence on normal perfusion . The loss of plaque volume after PCI was also the only significant predictor of CK-MB post-procedural release in a multiple regression model performed on 110 consecutive patients with stable or unstable angina . Tanaka et al reported that a pre-procedural “pool-like” aspect of culprit lesion at intravascular ultrasonography is an independent predictor of no-reflow in primary PCI, as a potential source of emboli. Kotani et al described consistently a higher quantity of plaque-derived debris and cholesterol crystals in coronary blood samples of patients with no-reflow compared to subjects with good post-procedural results.
Taken as a whole, these data support a causal link between PCI-induced atheroembolism - involving both thrombotic material and the lipid core of a ruptured fibroatheroma - and post-procedural microvascular obstruction.
Although angiographically difficult to detect, microembolic phenomena are highly frequent and similarly imply important biological consequences , , . Rogers et al showed that over 65% of iatrogenic emboli can be smaller than 56 μm in diameter on a case series of 64 venous graft interventions with distal protection. Quan et al reported the size distribution of PCI-induced emboli on native coronary arteries, showing a percentage of microemboli ranging from 40% to 65% according to the protection device used.
Iatrogenic microembolization, especially in ACS, is thus definitely conspicuous and not rare, and is related to malignant arrhythmias and contractile dysfunction , , which often extends beyond necrotic areas as a result of inflammatory response , , typically triggered by microembolic phenomena , , , .
Embolic events and no-reflow
No- or slow-reflow phenomena after PCI, defined as a suboptimal or absent coronary flow notwithstanding epicardial vessel's successful treatment, complicate a non-negligible proportion of coronary interventions , and predict worse clinical outcomes , both after urgent (reperfusion no-reflow) and elective (interventional no-refow) PCIs.
Microvascular obstruction is the leading underlying cause and embolic events are often a pivotal trigger, although MVO can be the result of different, synergic mechanisms . Indeed, in animal models, a transitory occlusion of a normal coronary artery with an external device - followed by arterial walls´ release - can end up in a no-reflow in absence of embolic events through the induction of arterial spasms, myocardial inflammation, microvascular compression by the oedema, endothelial swelling and intra-myocardial hemorrhage.
A close link between embolic phenomena and MVO is however widely recognized and motivates efforts to prevent such unfavorable events during PCI.
The assessment of microvascular patency is nowadays considered of paramount importance, together with plaque characterization and final indices of myocardial reperfusion as predictors of future clinical outcomes.
Invasive and non-invasive techniques aim at predicting the probability of embolic events during PCI, exploring microvascular patency afterwards and relate it to reperfusion success.
Non-invasive assessment of microvascular obstruction and myocardial reperfusion:
ST-segment resolution on 12-lead electrocardiography
In patients referred for primary PCI during ST-Elevation Myocardial Infarction (STEMI), ST-segment resolution on 12-lead electrocardiography is a simple and inexpensive method to assess post-procedural myocardial reperfusion success after primary PCI or fibrinolysis . Several studies have shown a consistent relationship between the degree of ST-segment resolution and cardiovascular mortality. After reperfusion therapy, complete ST-segment resolution implies good microvascular perfusion with a more favorable prognosis, whereas sustained or additional elevation of ST segment is associated with worse functional and clinical outcomes . More recently, residual ST-segment elevation after intervention instead of the percentage of ST-segment elevation resolution has also been validated to provide important prognostic information, obviating the need to compare pre- and post-reperfusion EKGs.
Biomarkers and soluble factors
Myocardial damage resulting from spontaneous or iatrogenic coronary microembolization is typically reflected by a transient elevation of creatine kinases and cardiac troponins , , , , , even in patients with baseline normal values referred for elective PCI. The issue is specifically addressed in the documents released by the ESC and by the ACC defining myocardial infarction subtypes , since post-procedural troponin elevation can predict a worse prognosis , , together with markers of inflammation such as C-reactive protein and IL-6 , , . Procedure-related myocardial injury is generally related to procedure complexity: direct stenting without balloon predilation attenuates microvascular dysfunction and troponin release , while more complex interventions with a higher number, duration, or pressure of balloon inflations or with atherectomy generally increase the incidence of post-procedural biomarkers’ elevation . Interpretation of further, post-procedural increases of markers of myocardial damage in the context of ACS is obviously more complex, as mostly related to the underlying spontaneous event.
Non-invasive Imaging
After an acute coronary syndrome, post-PCI Single Photon Emission Computed Tomography (SPECT) can help visualize microvascular obstruction as a lack of distribution of macroaggregated albumin microspheres even in the presence of a normally restored epicardial blood flow .
Similarly, myocardial contrast echocardiography uses microbubbles as a contrast dye to trace the intravascular space of normally-perfused territories, with the result that areas of MVO are visualized as a minus . Myocardial contrast intensity reflects the concentration of microbubbles within the myocardial capillaries and, therefore, myocardial perfusion volume. In patients undergoing PCI for AMI, contrast echocardiography has been used to assess the restoration of microvascular integrity, with an impact on left ventricular reverse remodeling after reperfusion.
Nowadays, however, contrast-enhanced magnetic resonance imaging (MRI) has become the most widespread and standardized non-invasive technology for the assessment of MVO , , . The feasibility of such assessment and its prognostic significance has been described since 1998 .
As a general principle, in post-AMI patients gadolinium chelates tend to accumulate in the necrotic area, as a result of their extravasation among ruptured myocytes. Cellular degradation increases the permeability and the overall amount of the extravascular space, with an augmented distribution volume for the contrast agent. Gadolinium is slowly washed out from the infarcted tissue in comparison to the surrounding viable myocardium. The final effect is that late T1-weighted MRI acquisitions, recorded 10-15 min after contrast administration, visualize the infarct area as bright and hyper-enhanced (late enhancement), surrounded by dark myocardial tissue where contrast wash-out has already been achieved.
If in the core of the infarcted area there is a zone of MVO, gadolinium delivery is only partial or totally absent, thus creating a “black” (hypo-enhanced) shadow inside the bright scar, as a reflection of a persistent hypoperfusion , , , , , .
MRI as a gold standard for the definition of myocardial scar and microvascular obstruction in nowadays supported by large evidences. A greater reduction of plaque volume during PCI has been related to the detection of new late gadolinium enhancement at MRI after 24 hours , , as a result of microembolization. In the experimental settings of pigs with induced coronary emboli, microinfarcts exceeding 5% of the myocardial mass were shown to be visible at late-enhancement MRI , , , suggesting a high sensitivity of the technique.
New radioactive (including PET), magnetic, and ultrasonic imaging modalities are under continuous development and will provide further insights on microvascular obstruction in the upcoming years , , .
Invasive risk prediction and assessment of MVO
Intravascular Ultrasonography (IVUS)
IVUS examination not only can visualize the necrotic lipid core and the thin fibrous cap of the plaque, predisposing to microembolization , , , , but can also estimate plaque volume variation and plaque cavity after dilation, which is related to the washout of debris into the microcirculation (see above) . Microemboli can be visualized and counted as high-intensity signals with a doppler wire; their count correlates with troponin release and is inversely related to post-procedural coronary blood flow and myocardial inotropic recovery , , .
Optical coherence tomography (OCT)
Optical Coherence Tomographic (OCT) has been used to analyze the characteristics of different types of coronary thrombi, and images have been related to post-mortem histologic examinations . Red thrombi were then retrospectively identified as high back-scattering protrusions inside the lumen of the artery with signal-free shadowing, while white thrombi were described as low back-scattering elements. Polarization-sensitive optical coherence tomography can provide information on the microstructural, compositional, biochemical and biomechanical features of coronary lesions, of intraluminal thrombotic debris and of stents.
Angiographic indices of microvascular obstruction
TIMI flow grade, developed by the TIMI group more than 20 years ago, has been widely adopted in the contemporary practice to define epicardial blood flow on a semi-quantitative scale, from 0 to 3. After PCI, a TIMI 3 flow is considered as an optimal, brisk coronary flow, while TIMI 0 to 2 results are expression of a no-reflow or a slow-flow phenomenon, which can be due to microvascular obstruction if an epicardial mechanical obstruction potentially impairing contrast progression can be excluded (i.e. flow-limiting dissection, significant residual stenosis). TIMI flow sensitivity for the diagnosis of microembolism is however low, since coronary flow in the epicardial artery can be normal notwithstanding microvascular obstruction. Moreover, TIMI flow grade assessment is highly variable, as shown by the discrepancies between on-site and core laboratory readings .
TIMI frame count has been defined as the number of angiographic frames (acquired at 30 frames per second) required for contrast to fill the epicardial vessel and reach a standardized distal landmark , . A low TIMI frame count indicates rapid coronary perfusion, while a high TFC is typical of perfusion impairment. A correction factor is required to compensate for the longer length of the LAD, compared with the LCX and RCA (the total number of frames is divided by a 1.7 fixed coefficient). After adjustment for vessel length the frame count number is termed as “corrected TFC” (cTFC). In absence of mechanical obstructions of the epicardial artery, microvascular obstruction is traditionally defined when cTFC is above 28 frames . cTFC has the advantage of being moderately reproducible, but as TIMI flow has low sensitivity and specificity for the assessment of MVO.
Myocardial blush grade (MBG) is a semi-quantitative assessment of the intensity of the induced radiopacity of myocardial tissue - achieved after the injection of the contrast dye in the epicardial vessel - and of the rapidity of enhancement wash-out in terms of heart cycles. MBG is considered a surrogate index of microvascular patency. An optimal myocardial perfusion is generally identified by a more intense blush and a rapid wash out of the contrast dye. The blush is assessed on angiographic runs and classified into 4 grades, from 0 to 3. In the absence of mechanical epicardial obstruction, a MBG 0/1 suggests microvascular obstruction .
Invasive functional assessment of microvascular patency
Coronary flow reserve (CFR) is analyzed with a 0.014-inch Doppler-tipped guidewire positioned in the target coronary artery . After induction of hyperemia by intracoronary or intravenous adenosine, CFR is calculated as the ratio of hyperemic-to-baseline peak velocity. A CFR of less than 2.0 is usually considered abnormal but can be equally impaired by epicardial obstruction, which needs to be excluded in advance.
The Index of microcirculatory resistance (IMR) requires a coronary pressure wire with a sensor/thermistor on its tip that can measure simultaneously intracoronary pressures and hyperemic mean transit time, which is proportionally related to absolute coronary blood flow. IMR is the ratio between these two measures and specifically addresses the definition of microvascular resistance, regardless hemodynamic conditions and epicardial vessel obstruction .
As wide efforts have been made to predict the risk and recognize MVO, comparable resources have been invested to find procedural techniques to prevent or manage coronary embolic events as a strategy to preserve microvascular patency and warrant better, post-procedural clinical outcomes.
Such interventional, alternative approaches are the core of this chapter.
Procedural strategies to prevent or manage coronary embolization
Several strategies have been developed and tested in clinical trials to prevent or manage coronary embolization during PCI, not only in the context of acute myocardial infarction. Controversial evidences have been produced for thrombectomy techniques and for coronary protection devices (Table 1), engineered to capture embolic fragments and remove them before they impair coronary microcirculation.
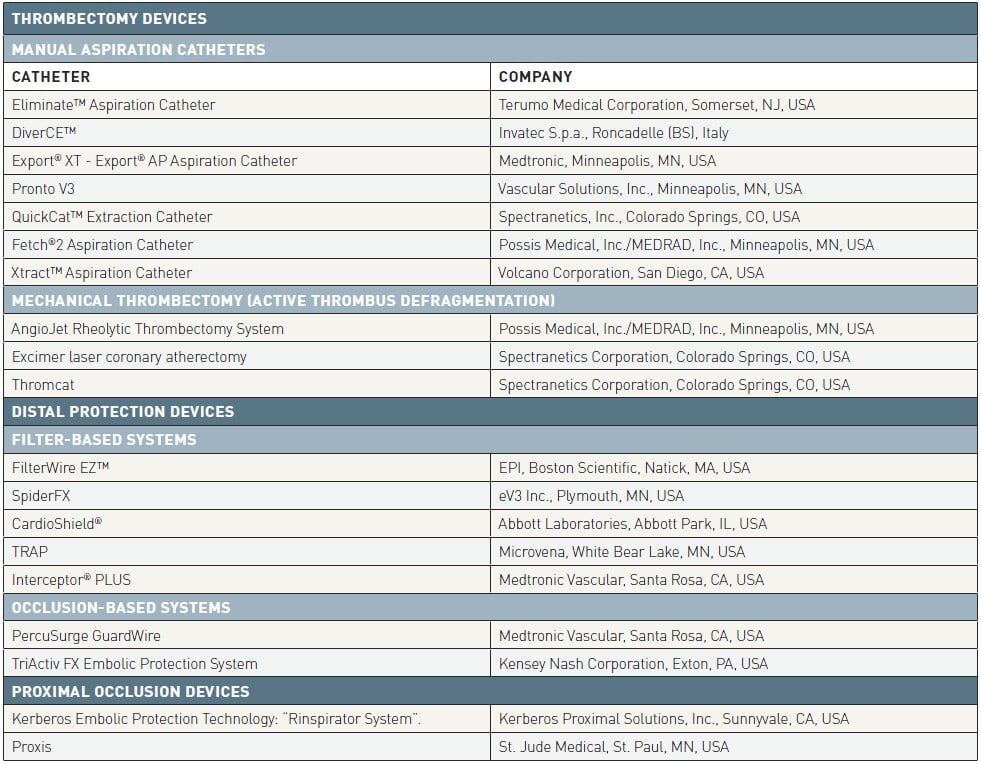
Table 1
Proxis system, technical specifications (St. Jude Medical).
As a general principle, occlusion devices are meant to be deployed distally or proximally to the target lesion, and are intended to temporarily stop bloodstream during intervention to allow debris removal from the vessel before flow is restored. Filters are deployed distally in culprit vessel and removed after PCI with their embolic content. Thrombectomy devices, instead, aim at thrombus removal before lesion manipulation, to decrease its embolic potentialities.
Coronary Thrombectomy
Thrombectomy devices aim at thrombus extraction before standard PCI is performed (or during lesion treatment, as a bailout strategy in case of migration). Thrombectomy can be generally achieved by the manual aspiration of the clot (which is usually referred as “manual thrombectomy”) or by a motorized device disrupting and removing thrombotic particles (“mechanical thrombectomy”).
The history of thrombectomy dates back to the 1980s, when the first attempts to manage large thrombus burden were described, but techniques and devices are still evolving since controversial data about their efficacy and safety profile have emerged.
Manual Thrombectomy
Several catheters for manual aspiration thrombectomy are currently available, sharing the same rationale. In general terms, a catheter with a central aspiration lumen is advanced in proximity of the target thrombus on a standard 0.014” coronary wire and is then externally connected to an aspiration syringe. Continuous suction mobilizes and aspirates thrombotic debris, reducing intra-luminal content. Some general recommendations on how to perform manual aspiration have been proposed, in order to increase its efficacy and prevent complications:
- Aspiration catheters should always be flushed with heparinized saline solution, to avoid air embolization during maneuvers
- Aspiration should begin 2 cm proximal to the target lesion, then thrombectomy catheter should be gently pushed forwards across the clot during continuous suction. Retrograde thrombectomy technique, with aspiration starting after the lesion has been crossed, is not recommended, as a source of potential embolism
- Slow movements should be granted since they are less likely to provoke thrombus dislocation
- In case of back-bleeding interruption during aspiration, slow forward and backward movements can help aspiration catheter disengage from arterial wall or plaque
- Use of a second or third aspirating syringe to prolong suction is generally recommended
- Suction should be kept while thrombectomy catheter is removed, to prevent debris disengagement from its tip during retrieval, and a deep engagement of the guiding catheter at coronary ostium should be granted, to avoid embolization of thrombotic debris in the aorta when the distal tip of thrombectomy device enters the guide
- A vigorous flush of the guiding catheter after each passage is mandatory, to remove thrombotic remnants from the Y-connector
If there is no reduction in thrombus burden or if the aspiration catheter itself cannot cross the lesion, predilatation with a small balloon is an option but it should take into account an increased possibility of embolization.
For the treatment of larger vessels 7-8 Fr -compatible catheters are available, and can be unsurprisingly more effective.
The most widely used manual thrombectomy devices are reported hereafter.
Eliminate™ Aspiration Catheter (Terumo Interventional System) (Figure 1)

Figure 1
Eliminate (Terumo) aspiration catheter.
This product has been designed to provide the best combination of crossing performance, kink resistance and thrombo-aspiration capability. Beyond its technical characteristics, the device has an historical importance as the catheter of choice in randomized clinical trials (see forwards).
Key structural features (Table 2 and Table 3)
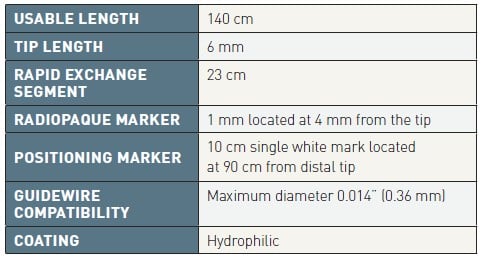
Table 2
Eliminate (Terumo) general specifications.
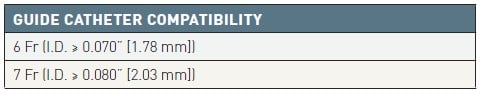
Table 3
Eliminate (Terumo) item specifications.
- Large extraction lumen and rounded short tip design, for enhanced aspiration capability
- Short and thin distal marker, for ease of visualization on radioscopy
- Complete kit for single-handed operator use
- Pre-loaded stylet, fully braided shaft and 23-cm-long rapid exchange segment for improved deliverability
Trials
TROFI
This was a multicentre, prospective, randomized controlled trial enrolling 141 STEMI patients randomly assigned to receive primary PCI with vs. without preliminary thrombectomy. The primary end-point was the minimum flow area reached immediately after primary PCI, assessed by OCT imaging and defined as the difference between re-opened vessel area and intra-luminal residual defects. The trial failed to demonstrate a significant difference between the two arms (7.08±2.14 mm2 vs. 6.51±1.99 mm2, p = 0.12), with a post-hoc analysis suggesting a possibly better result on patients with large thrombus burden (TIMI Thrombus Grade, TTG≥4), advocating further evidences.
TASTE ,
This multicentre, prospective, randomized controlled trial included 7,244 STEMI patients randomly assigned to receive thrombus aspiration followed by PCI or PCI alone, and was powered to verify the clinical impact of manual thrombectomy with Eliminate catheters in terms of 30-day all-cause death. The primary endpoint occurred in 2.8% of patients treated with thrombectomy and in 3% of those allocated in PCI-only group, without any significant difference (HR 0.94; 95% CI 0.72-1.22; p=0.63). As far as secondary endpoints, the rate of hospitalization for recurrent myocardial infarction at 30 days was respectively 0.5% and 0.9% in the two groups (HR 0.61; 95% CI 0.34-1.07; p = 0.09) and the incidence of stent thrombosis was 0.2% and 0.5% (HR 0.47; 95% CI 0.20-1.02; p = 0.06). Mortality did not differ at one year follow-up (5.3% vs. 5.6%; HR 0.94; 95% CI 0.78-1.15; p = 0.57), as also hospitalization for recurrent myocardial infarction (2.7% vs. 2.7%; HR 0.97; 95% CI 0.73-1.28; p=0.81) and stent thrombosis (0.7% vs. 0.9%; HR 0.84; 95% CI 0.5-1.4; p=0.51). Results were consistent among all pre-specified subgroups of patients, stratified by thrombus burden and by pre-PCI coronary blood flow.
DiverCE™ (Invatec S.p.a., Roncadelle [BS], Italy) (Figure 2)
Figure 2
DiverCE (Invatec) manual aspiration catheter.
Key structural features
This rapid exchange clot extraction catheter, available in 6 and 7 Fr-compatible sizes, has a 1.5 mm in diameter distal tip with a 1-mm proximal radiopaque marker, and has been developed in 2 versions:
- with side holes on the tip, for fresh thrombus removal (<6h from symptoms onset);
- with a central lumen exclusively open on the tip, to aspire organized thrombi (≈ 6-24 h post symptom onset).
The flexible shaft has a distal hydrophilic coating and an enlarged lumen to increase aspiration performances.
Trials
REMEDIA
The Randomized Evaluation of the Effect of Mechanical Reduction of Distal Embolization by Thrombus Aspiration in Primary and Rescue Angioplasty (REMEDIA) trial was a single-center, prospective, randomized study designed to assess the safety and efficacy of the DiverCE catheter for thrombus aspiration in STEMI patients. The co-primary endpoints were a myocardial blush score ≥2 and a ST-segment resolution >70%. Patients who were randomly assigned to thrombus aspiration showed better angiographic and electrocardiographic results in comparison to direct PCI (post-procedural MBG ≥2 68.0% vs. 44.9%, OR 2.6, CI 95% 1.2-5.9, p = 0.02; ST resolution ≥70% 58.0% vs. 36.7%, OR 2.4, 95% CI 1.1 to 5.3, p = 0.034). Clinical outcomes were analyzed as secondary endpoints without any significant difference, given the small sample size.
PIHRATE
Patients with STEMI <6 hours from onset of symptoms and an occluded infarct-related artery at baseline angiography were randomized to aspiration thrombectomy followed by direct stenting (n=100) or standard balloon predilatation followed by stent implantation (n=96). The primary endpoint of the study was ST-segment elevation resolution >70% 60 minutes after intervention. Aspiration thrombectomy success rate, defined as crossing of the lesion with thrombus reduction and flow restoration, was high (91%). Difference in ST segment resolution ≥70% after 60 minutes was not statistically significant (53.7% vs. 35.1%, p=0.29), but a significant difference was observed immediately after PCI (41% vs. 26%, p<0.05) as a myocardial blush grade ≥3 (76% vs. 58%, p<0.03) suggesting a faster recovery in the aspiration group. Once more, clinical secondary endpoints didn’t show any difference, calling for adequately powered studies.
Export® XT/AP aspiration catheters (Medtronic, Minneapolis, MN, USA) (Figure 3)
Figure 3
Export XT (Medtronic) catheter tip design.
Export XT was the first thrombectomy device released by Medtronic, a 6-7 Fr compatible, rapid exchange system cleared for use in the U.S. in 2006. Export AP is the newer, currently available iteration of the device.
Key structural features
- Full-wall braiding technology helps to reduce the incidence of kinks and to improve overall deliverability, eliminating segment joints and weak points in the catheter with a seamless transition of shaft stiffness from one end to the other
- Proximal shaft braiding grants higher support while distal shaft braiding enhances flexibility
- Soft material and hydrophilic increase the lubricity of the tip, whose design aims at increasing deliverability and optimizing thrombus aspiration.
Trials
Export devices have been widely tested in clinical trials through the years. Some of these studies, like EXPORT, EXPIRA and TAPAS, have marked the success of manual thrombectomy for the treatment of STEMI patients, showing improved myocardial reperfusion and suggesting encouraging results on survival. Such data have been largely overcome by the most recent, large RCTs, mainly performed with the same device and adequately powered for clinical outcomes, showing no benefit in a routinely use of aspiration thrombectomy in primary PCI. The same aspiration catheter was used in a subgroup of patients of the TASTE trial , .
EXPORT
This single-center, prospective, randomized trial aimed at assessing the safety and efficacy of thromboaspiration with Export device in primary PCI in 250 patients with AMI. For the primary endpoint (the combined rate of myocardial blush grade = 3 and/or ST-segment resolution >50%) thromboaspiration followed by stenting was superior to conventional stenting (85.0% vs. 71.9%, p=0.025). Immediately after procedure, the rate of myocardial blush grade 3 was 35.8% for primary thromboaspiration followed by stenting versus 25.4% for conventional stenting (p=0.094). At 60 minutes post procedure, the rate of ST-segment resolution >50% was 73.5% for primary thromboaspiration followed by stenting versus 64.8% for conventional stenting (p=0.218). Thromboaspiration was associated with a significantly lower post-procedural TIMI frame count (20±14.9 vs. 22.8±14, p=0.02). At 30 days, no significant differences with respect to the rate of major adverse cardiac and cerebral events was noticed between the two groups.
EXPIRA
This single-center trial tested the impact of manual thrombectomy as adjunctive therapy in primary PCI in terms of rate of post-PCI MBG≥2 and 90-min ST-segment resolution ≥70% (co-primary endpoints). A subgroup of patient underwent a contrast-enhanced MRI assessment to assess infarct size and microvascular obstruction. 175 anterior STEMI patients were randomly assigned to standard percutaneous coronary intervention (n=87) vs. thrombectomy + PCI (n=88). Myocardial blush grade ≥2 and ST-segment resolution occurred more frequently in the thrombectomy group (88% vs. 60%, p = 0.001; and 64% vs. 39%, p = 0.001). In the acute phase, microvascular obstruction extent was significantly lower in the thrombectomy group and, at 3 months, infarct size was significantly reduced for the same patients in comparison to baseline. A hypothesis-generating lower incidence of cardiac death in the thrombectomy group (0% vs. 4.6%, log-rank test p = 0.02) was observed at 9 months, advocating adequately powered studies with strong clinical endpoints.
TAPAS ,
The TAPAS trial randomized in a single center 1,071 patients to thromboaspiration + PCI vs. primary PCI alone. Aspiration was considered successful if there was histopathological evidence of atherothrombotic material, detected in 72.9% of patients. The primary endpoint was a myocardial blush grade of 0 or 1 (as a marker of absent or minimal myocardial reperfusion). Electrocardiographic signs of reperfusion and clinical outcomes were also assessed as secondary endpoints. The primary EP occurred in 17.1% of the patients in the thrombus-aspiration group and in 26.3% of those in the conventional-PCI group (P<0.001). Complete resolution of ST-segment elevation occurred in 56.6% and 44.2% of patients, respectively (P<0.001). At 30 days, the rate of death in patients with a myocardial blush grade of 0 or 1, 2, and 3 was 5.2%, 2.9%, and 1.0%, respectively (P=0.003), and the rate of adverse events was 14.1%, 8.8%, and 4.2% (P<0.001). Consistently, at 1 year cardiac death occurred in 6.7% of patients in the conventional PCI group and in 3.6% in the thromboaspiration group (hazard ratio [HR] 1.93; 95% CI: 1.11–3.37; p=0.020). The composite endpoint of one-year cardiac death and non-fatal re-infarction occurred in 9.9% of patients in the conventional PCI group and in 5.6% of patients in the thromboaspiration group (HR 1.81; 95% CI: 1.16–2.84; p=0.009). Authors concluded that thromboaspiration before stenting significantly improved angiographic and electrocardiographic indices of successful reperfusion. This was translated into improved 1-year clinical outcomes after PCI (Figure 4).
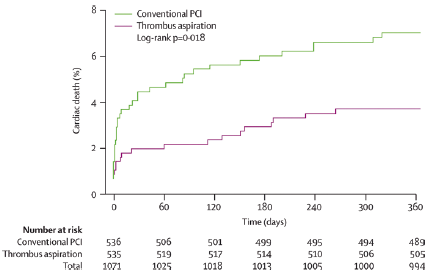
Figure 4
Kaplan-Meier curve for cardiac death at 1-year follow-up in the TAPAS trial.
TAPAS trial drove the results of a subsequent meta-analysis including 25 RCTs in which patients were randomized to receive mechanical or manual thrombectomy vs. standard PCI. A clinical benefit in terms of all-cause mortality was shown for aspiration thrombectomy - but not for mechanical thrombectomy - over PCI alone (2.7 % vs. 3.9%, RR 0.71, 95% CI 0.51-0.99, p=0.049). Analogous results were observed in terms of MACE (10.8 vs 14%, RR0.76, 95% CI 0.363-0.92, p = 0.006). Concerns were arisen about a trend towards a higher incidence of cerebrovascular events, advocating properly sized clinical trials specifically addressing this issue.
INFUSE-AMI
The trial randomized 452 anterior STEMI patients, in 37 sites across 6 Countries, in an open-label, 2×2 factorial design to intracoronary abciximab vs. no abciximab and to manual aspiration thrombectomy vs no thrombectomy. Infarct size at 30 days assessed by cardiac magnetic resonance imaging (cMRI) in the aspiration vs no aspiration groups (pooled across the abciximab randomization) was addressed as a secondary endpoint. The primary endpoint of the study was infarct size in abciximab vs. no-abciximab group (pooled across thrombus aspiration). 174 and 179 patients were randomized to manual aspiration vs no aspiration, respectively. While intracoronary abciximab showed a significant reduction in 30-day infarct size (median, 15.1%; interquartile range [IQR], 6.8%-22.7%; vs 17.9% [IQR, 10.3%-25.4%]; p: 0.03), a beneficial effect was not detected for aspiration thrombectomy (for infarct size: median 17.0% [IQR, 9.0%-22.8%] vs 17.3% [IQR, 7.1%-25.5%]; similar results for absolute infarct mass and wall motion score index). Investigators concluded that in patients with anterior STEMI presenting early after symptom onset and undergoing primary PCI (with bivalirudin anticoagulation), infarct size at 30 days was significantly reduced by intracoronary abciximab bolus but not by manual aspiration thrombectomy. Additional efficacy endpoints included parameters of angiographic reperfusion, ST-segment resolution at 1-hour, 30 days and 1-year and the rate of MACCE (death, reinfarction, stroke, or clinically driven target vessel revascularization) and bleedings at 30 days, once more without any evidence of potential benefit for manual aspiration.
TOTAL
To specifically address the potential clinical benefit of manual thrombectomy in the context of primary PCI – after the controversial results of previous studies - the TOTAL trial randomized 10,732 STEMI patients to receive either routine manual thrombectomy + standard PCI or PCI alone, and was powered for a primary composite endpoint of cardiovascular mortality, recurrent myocardial infarction, cardiogenic shock or NYHA functional class IV within 6-month. The major safety endpoint was stroke within 30 days. Random assignment to groups was performed upon STEMI diagnosis on ECG, regardless intracoronary thrombotic burden. Primary endpoint rates were similar in patients who received thrombectomy as compared to those undergoing PCI alone (6.9% vs. 7%; HR 0.99; 95% CI 0.85-1.15; p=0.86). 30-day stroke rates, instead, were significantly higher in patients treated with thrombectomy (0.7% vs. 0.3%; HR 2.06; 95% CI 1.13-3.75; p=0.02), arising substantial safety concerns on routine manual aspiration (Figure 5).
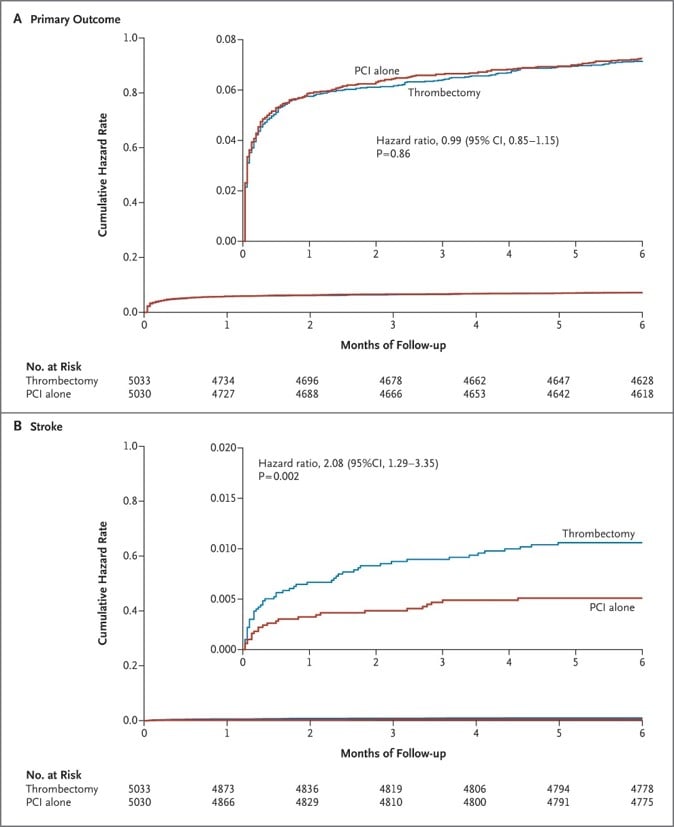
Figure 5
Efficacy and safety of aspiration thrombectomy in TOTAL Trial. On top, event-free survival (cardiovascular mortality, recurrent myocardial infarction, cardiogenic shock or NYHA functional class IV) after primary PCI with and without trombectomy. Below, occurrence of stroke within 30 days.
Trial results were consistent across pre-specified subgroups, regardless intracoronary thrombotic burden (assessed by TIMI Thrombus Grade and TIMI flow after the first injection of contrast dye in the infarct-related artery).
Subsequent sub-studies on TOTAL population addressing angiographic endpoints confirmed the absence of a significant benefit in terms of TIMI flow and MBG, as surrogates of successful reperfusion. ,
Pronto V3 (Vascular Solutions, Inc., Minneapolis, MN, USA)
The PRONTO V3 extraction catheter is a dual lumen rapid exchange catheter.
Key structural features
- A larger extraction lumen;
- A rounded distal tip with a protected, sloped opening, to facilitate advancement and maximize extraction of thrombus;
- A proximal stiff region and a distal flexible region with a lubricious hydrophilic coating to increase deliverability.
A 70 µm filter basket included, to filter the blood removed during the procedure for laboratory analysis.
Trials
DEAR-MI
DEAR-MI was a 1:1 open-label study randomizing 148 AMI patients within 12 hours of symptom onset to either thromboaspiration or standard PCI. All of them underwent stenting and were concomitantly treated with abciximab. After adjusting for confounding factors, multivariate analysis showed thromboaspiration to be an independent predictor of complete ST-resolution and optimal myocardial blush.
The same aspiration catheter was used in a subgroup of patients of the TASTE trial , .
QuickCat™ extraction catheter (Spectranetics, Inc., Colorado Springs, CO, USA)
The QuickCat™ Extraction Catheter is a disposable dual lumen catheter compatible with 6 Fr guiding catheters.
Structural features
- Radiopaque marker 1 mm proximal to the tip;
- Designed with a flexible, hydrophilic-coated distal end and an increasingly stiffer proximal end for improved pushability;
- Low crossing profile with a consistent extraction lumen.
Trials
There is no RCT exploring the efficacy and safety profile of the device in the context of acute coronary syndromes.
Fetch®2 aspiration catheter (Possis Medical, Inc./MEDRAD, Inc., Minneapolis, MN, USA) (Figure 6)
Figure 6
Fetch aspiration catheter (Medrad).
The Fetch2 Aspiration Catheter provides good handling characteristics with a small crossing profile. It is designed for enhanced trackability and performance, for small or discrete thrombus volume cases.
Structural features
- Flexible, hydrophilic-coated distal shaft, enhancing catheter's pushability
- Convex tip, minimizing vessel trauma and enhancing catheter’s ability to navigate narrow vessels with tight bends.
Trials
There is no RCT exploring the efficacy and safety profile of the device in the context of acute coronary syndromes.
Xtract™ aspiration catheter (Volcano Corporation, San Diego, CA, USA)
The Xtract aspiration catheter is a single-user, 0.014” guidewire compatible, intravascular extraction and aspiration catheter, available in 4.2 Fr and 5.8 Fr with 0.014” guidewire compatibility.
Structural points
- Large, single lumen design increasing suction duration (>11 seconds) and efficiency.
Trial
The Xtract™ aspiration catheter registry study was launched in 2006 and completed in 2008. Results have not yet been reported.
Thrombectomy devices
Emerging strategies for manual thrombectomy
NeVA stent retrievers (Figure 7)
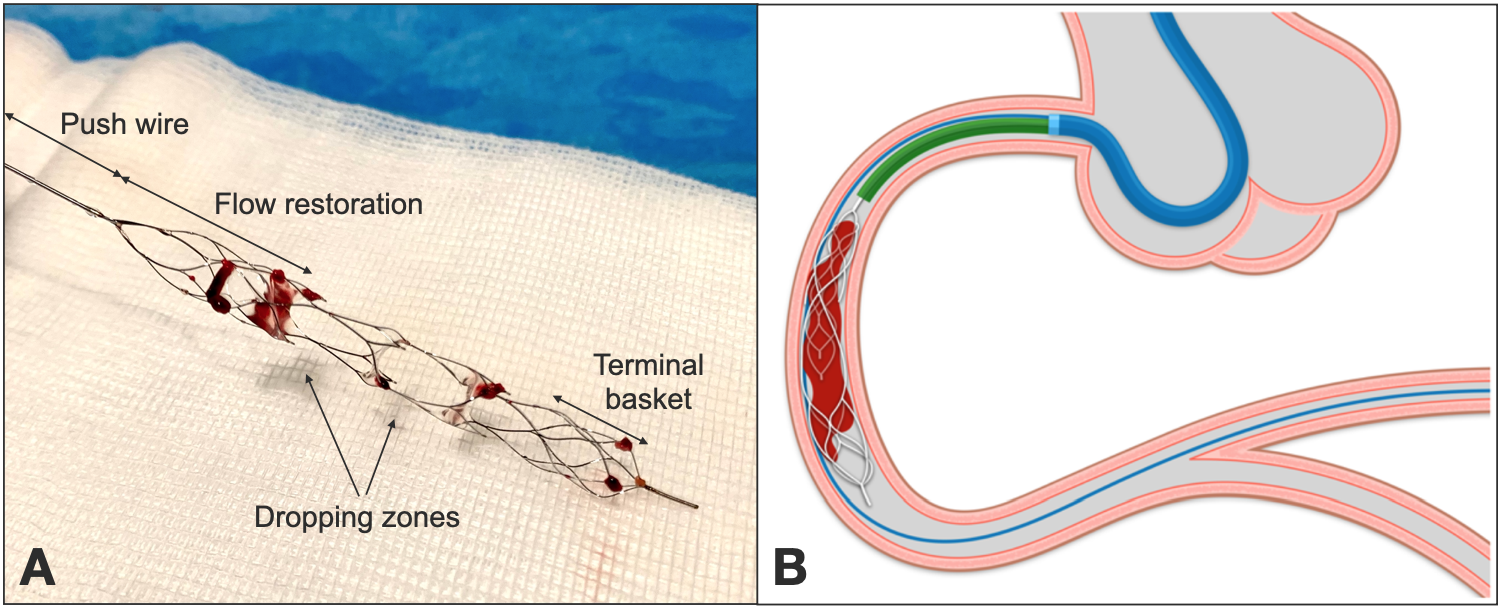
Figure 7
Panel A shows a NeVA stent retriever with macroscopic thrombotic fragments after coronary thrombectomy. The push wire is attached to the flow restoration zone; two dropping zones allow thrombus capture and are followed by a terminal basket.
On Panel B system configuration during thrombectomy: a telescopic catheter (in green) is advanced on the push wire close to the proximal edge of the stent retriever, to maximize clot aspiration. Stent retriever is then withdrawn together with the telescopic catheter (and the embedded clot) during continuous aspiration on the guide.
Stent retrievers are manual thrombectomy devices (MTD) with a self-expandable nitinol stent attached to a push wire, engineered to achieve immediate flow restoration in occluded vessels upon deployment, capture intraluminal thrombus and remove it with stent withdrawal. After both angiographic and clinical favorable outcomes have been shown, such devices have been widely used and are nowadays a standard of care for stroke interventions – where the clot is usually the leading actor underlying vessel occlusion.
During thrombectomy, the clot enters the device through dedicated dropping zones, identified on fluoroscopy by radiopaque markers. After clot capture, retrieval maneuvers are performed during continuous aspiration to prevent debris embolization.
The technique has recently been readapted to be used in ACS patients with large thrombus burden. In a first-in-human case series of 61 consecutive patients , flow restoration upon stent retriever´s deployment was achieved in 85% of cases, with an immediate TIMI 3 flow in 74% of patients showing TIMI 0 at baseline. The use of the MTD in coronary arteries was safe, without major procedural complications (i.e. dissections, perforations), procedure-related cerebrovascular events and with a single case of thrombotic embolization in a side branch during retrieval maneuvers, which was however successfully resolved with the same thrombectomy technique. Aspiration modality was optimized on a bench model and routinely implemented across the case series.
A multicenter, prospective, randomized controlled study is currently ongoing, to assess the safety and effectiveness of this new treatment modality in patients with STEMI and LTB in comparison to standard PCI (NCT04969471).
Mechanical Thrombectomy
In comparison to manual thrombectomy, which is mainly achieved by aspiration through dedicated catheters, mechanical thrombectomy is performed with mechanical devices aiming at thrombus fragmentation and removal.
AngioJet Rheolytic thrombectomy system (Possis Medical, Inc./MEDRAD, Inc., Minneapolis, MN, USA) (Figure 8 and Figure 9)
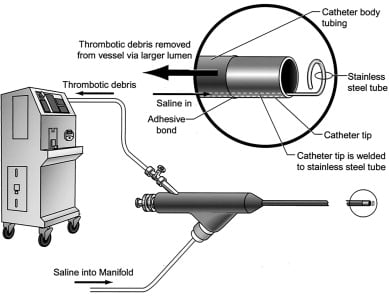
Figure 8
AngioJet Rheolytic Thrombectomy System (Medrad).
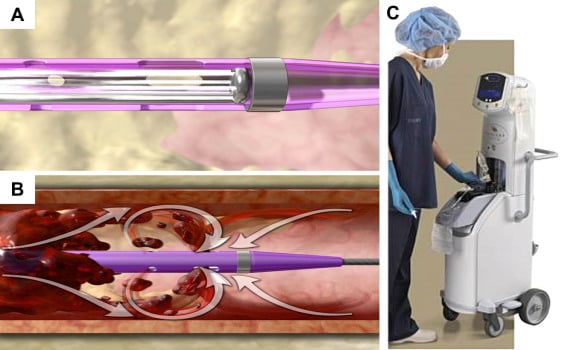
Figure 9
Retrograde saline high-velocity flow in Angiojet system´s tip (A) to achieve vacuum and aspiration thrombectomy according to Bernoulli´s principle (B). AngioJet system console (C).
Angiojet system was the first device available on the market to perform mechanical thrombectomy. Possis Corporation developed many industrial uses for this technology before the same principles could be readapted to a miniaturised thrombectomy device. Large industrial water jet systems were initially designed to cut difficult materials, including glass, titanium and stone, relying on the special cutting properties of water jets, i.e. the absence of significant heat or sparks . Possis Medical began developing the AngioJet RT System in 1989, initially for atherectomy applications, then with a focus on thrombectomy , . Extensive preclinical testing , and clinical trials have been performed through the years on a wide span of clinical contexts, exploring its potential benefit for the treatment of acute coronary syndromes, peripheral artery disease, pulmonary embolism.
Structural features and mechanism of action
The AngioJet Rheolytic Thrombectomy (RT) System mainly consists of three components: a drive unit console, a pump set and a disposable catheter. The drive unit console controls the whole system, providing a feedback to the user for RT set-up and operation.
The pump set includes a high-pressure pulsatile pump, used to generate the high-pressure flow that is necessary for thrombus fragmentation and aspiration. Pressurized saline is delivered to the catheter tip by the pump through a high-pressure conduit, which is contained within a stainless-steel hypotube terminating distally in a closed loop, entirely enclosed inside catheter’s distal end. The distal loop receives fluid from the pump at 7,000 - 12,000 psi. This loop has tiny jet orifices (50 µm in diameter) directed retrogradely towards the catheter manifold. Backwards flow, through these tight holes, increases in velocity (16,000 cm/sec) at the price of a dramatic pressure drop. This creates a near-perfect vacuum (-600 mm Hg), producing aspiration forces by Bernoulli principle inside the aspiration catheter. Thrombus suction, maceration and extraction is then achieved without any contact between high pressurized saline and vessel wall. Once aspirated through the side holes of the catheter, thrombotic debris are broken into small particles and evacuated through a tubing system, directed to a collecting bag.
Application of the RT catheter for power-pulse spray
Power-pulse spray infusion via the Xpeedior® RT catheter is a more recent innovation. A stopcock attached to the catheter manifold is used to close off the evacuation lumen and infuse fluids out of the distal catheter tip, with a lower delivery pressure ranging between 20-30 psi. This tool is used for a directed infusion of physician-specified fluids, generally thrombolytic drugs , meant to be delivered inside the clot and boost thrombus disaggregation.
Side effects
Hemolysis may occur during RT, due to the shear forces of the high velocity jets. This is more frequently observed during extended catheter run times, such as in peripheral procedures. Care should be taken to observe the recommended run times specified in the catheter instructions for use and to monitor indicators of hemolysis in post-procedural blood samples. RT-induced hemolysis tends to resolve within 24-48 hours and is not usually associated with relevant clinical sequelae, but cases of kidney and/or pancreatic failure have been described.
Moreover, the system can cause bradycardia and even asystole since hemolysis is related to adenosine release, especially when performed on the right coronary artery. Such procedures often require temporary right ventricular pacing, which could be however avoided by the routine administration of atropine as shown in the JETSTENT trial (see forward).
Tips and Tricks
- The XMI® and Spiroflex® RT catheters have a relatively low profile: delivery over a 0.014” moderate support guidewire is recommended;
- The RT catheter is preferably delivered without predilatation; however, low pressure predilatation with an undersized balloon (1.5-2 mm in diameter) may be performed if necessary;
- For coronary applications, catheter activation should start at least 1 cm proximal to the thrombus, to create a suction vortex prior to device advancement in a proximal-to-distal direction ; distal-to-proximal pullback techniques have been reported to treat saphenous vein grafts with large thrombotic burden and a free-floating distal tail ;
- Care should be taken to keep run times as low as specified in the catheter instructions for use to prevent hemolysis,
- Atropine injection, and in some cases insertion of a temporary pacing, is generally recommended during the treatment of the right coronary artery for a higher risk or brady-arrhythmias.
Trials
AiMI
AiMI was a multicentre, prospective, randomized clinical trial comparing AngioJet thrombectomy followed by PCI versus standard PCI in acute coronary syndromes presenting within 12h from symptom onset, regardless the presence of a visible thrombus at coronary angiography. The hypothesis was that rheolytic thrombectomy could reduce infarct size measured by sestamibi SPECT at 14-28 days. Actually, final infarct size was higher in the RT group compared with PCI alone (12.5±12.13% vs. 9.8±10.9%; p=0.03). Among secondary endpoints, TIMI flow grade 3 rate was lower in the RT group (91.8% vs. 97.0%; p=0.02) and there were no significant differences in terms of blush scores or ST-segment resolution. Moreover, thirty-day MACE were higher in the interventional group (6.7% vs. 1.7%; p=0.01), driven by a very low mortality rate in the control arm (0.8% vs. 4.6%; p=0.02). Authors concluded that despite effective thrombus removal, RT with primary PCI did not reduce infarct size or improve perfusion indices, ST-segment resolution, or 30-day MACE.
FLORENCE-ANGIOJET TRIAL
The Florence-AngioJet was a small randomized trial enrolling 100 STEMI patients with a mechanistic endpoint of early ST-segment resolution, corrected TIMI frame count, and infarct size assessed by technetium-99m sestamibi scintigraphy at 1 month. Patients randomized to thrombectomy before direct stenting had a higher rate of early ST-segment resolution (90% vs. 72%, p=0.022), a lower corrected TIMI frame count (18.2±7.7 vs. 22.5±11.0, p=0.032) and a smaller infarct size (13.0%±11.6% vs. 21.2%±18.0%, p=0.010) in comparison to direct stenting alone. At 1 month, no patient died or had reinfarction. 6-months clinical outcomes were similar in the two arms, with a mortality rate of 2% in both groups.
JETSTENT trial , ,
JETSTENT was a prospective, multicentre, randomized, two-arm trial which notably focused on AMI patients with angiographic evidence of high thrombus burden (TIMI Thrombus grade 3 to 5). Co-primary endpoints were early ST-segment resolution (STR) and 99mTc-sestamibi infarct size. Secondary endpoints were TIMI grade 3 flow, corrected TIMI frame count and Myocardial blush grade 3. A composite of major adverse cardiovascular events at 1, 6 and 12 months was explored as a secondary clinical endpoint. From December 2005 to September 2009 501 patients were randomly allocated to Rheolytic Thrombectomy (RT) before direct stenting or to DS alone. ST-segment elevation resolution occurred in 85.8% of patients assigned to thrombectomy and in 78.8% of patients randomly allocated to DS alone (p=0.043). Infarct size was smaller in the thrombectomy arm, but this difference did not reach statistical significance: 11.8% (IQR 3.15% to 23.70%) vs. 12.7% (IQR 4.75% to 23.30%), p=0.398. None of the 3 secondary angiographic endpoints differed significantly. By contrast, 1-month MACE rate was 3.1% in the thrombectomy arm and 6.9% in the DS alone arm (p=0.05), and the difference increased at 6-month (11.2% vs. 19.4%; p=0.011) and 1-year follow-up (14.9% vs. 22.7%, respectively; p=0.036).
VeGAS-2
This trial randomized 352 patients with large thrombus burden to rheolytic thrombectomy versus infusion of urokinase in the target vessel, showing a >50% reduction in MACE at 1 month in thrombectomy arm (16% and 33% respectively, p<0.001), primarily due to a reduction in non-Q-wave periprocedural myocardial infarction.
The controversial experimental results summarized in this paragraph have eventually hampered the diffusion of rheolytic thrombectomy in ACS. A meta-analysis of RCTs comparing manual and mechanical thrombectomy to standard PCI didn´t show any clinical benefit for the latter in terms of mortality and of MACE, overcoming the promising results of the JETSTENT trial. The abovementioned safety concerns restricted contemporary indications in ACS to a possible bailout option.
Excimer laser coronary atherectomy (ELCA; Spectranetics Corporation, Colorado Springs, CO, USA) (Figure 10)
.png)
Figure 10
Excimer Laser Coronary Atherectomy Ablation (ECLA) System [Spectranetics].
The terms “excimer” and “laser” are acronyms. Excimer is an acronym for excited dimer whereas laser stands for light amplification by stimulated emission of radiation . Laser energy is produced from the xenon chloride laser when hydrogen chloride gas is excited by electrical energy, and emits monochromatic, coherent light at a wavelength of 308 nm, with a pulse frequency of 25-80 Hz and a penetration depth <10-50 micron. This laser energy ablates inorganic material by photochemical mechanisms that involve the breaking of molecular bonds without generation of heat . The exact mechanisms for tissue ablation consist of a combination of photochemical, photothermal and photomechanical effects. When light is absorbed by tissues (and by thrombus), an induced pressure wave boosts fluid displacement, leading to bubble formation and collapse with the final effect of thrombotic debulking. ELCA was also described to harbor a “stunned platelet” phenomenon, altering aggregation kinetics rand thus reducing platelet aggregation .
Lasers were introduced in interventional cardiology in the late 1980s. Significant concerns soon emerged regarding laser-related complications, namely dissections and perforations , leading to a drastic decline in procedures in the early 1990s. Thereafter, an improvement in equipment and technique renewed interest in laser angioplasty.
Initial FDA approval of the excimer laser was based on results of the excimer laser coronary angioplasty (ELCA) registries: Spectranetics Laser Registry consisted of 2,432 patients with a mean age of 63 years. Clinical success was achieved in 2,168 of 2,432 patients (89%), consistent across all subgroups of complex coronary lesions. In another large multicentre registry 3,000 patients were enrolled, with consistent results. Major complications included death in 0.5%, emergency coronary bypass surgery in 3.8%, Q-wave myocardial infarction in 2.1%, and non-Q-wave myocardial infarction in 2.3%. Perforations occurred in 1% of lesions but decreased significantly from 1.4% in the first 2,592 lesions to 0.3% in the last 1,000 lesions. Coronary dissection occurred in 13% of lesions, permanent occlusions in 3.1%.
A major advance in excimer laser angioplasty occurred in 1995 with the advent of the saline infusion technique . Since blood and radiographic contrast media avidly absorb ultraviolet laser energy, inducing significant acoustic effects, tissue disruption, and possibly dissection, the adjunction of saline infusion had the aim of eliminating blood and contrast dye from the laser field and resulted in a significant decrease in iatrogenic dissections from 24% to 7%.
Device description
Over-the-wire (OTW) catheters consist of multiple optical fibers arranged concentrically around a central guidewire lumen. Rapid exchange (RX) catheters consist of optical fibers encased within a polyester shaft. The proximal portion of the shaft terminates at the laser connector, the distal portion terminates in catheter’s tip, with direct patient contact. A radiopaque marker is located on the distal end of the laser catheter to aid localization during fluoroscopy. The guidewire lumen begins at the distal tip and exits laser catheter 9 cm proximally. Optical fibers are enclosed eccentrically, to allow alignment with the lesion. A torque system extends from the torque handle, located at the y-adapter, through the entire 140 cm of the catheter. A mechanism within the torque handle limits possible turns to five full rotations in each direction, and the catheter is provided with an indicator displaying its range of motion. The torque response is 6:1 - six turns of the torque handle result in one 360° turn of the distal tip.
The Vitesse C catheters (Spectranetics Corporation, Colorado Springs, CO, USA) are 1.4 mm, 1.7 mm, and 2.0 mm concentric devices whereas the Vitesse E eccentric catheters are available in 1.7 mm and 2.0 mm sizes. Vitesse Cos device provides a wider ablation area and is available in 1.4 mm, 1.7 mm, and 2.0 mm sizes.
Mechanism of action
Multifiber laser devices transmit ultraviolet energy from the Spectranetics CVX-300® to the artery. Ultraviolet energy is delivered to the tip of the catheter to photo-ablate fibrous, calcific and atheromatous lesions. Energy photons cause molecular bond disruption at cellular level, without thermal damage on surrounding tissue.
The Spectranetics laser catheters have a lubricious coating, to ease their trackability through coronary vessels.
Trials on acute myocardial infarction
CARMEL trial
Topaz et al studied the safety and efficacy of excimer laser in AMI. In the Cohort Acute Revascularization in Myocardial Infarction with Excimer Laser (CARMEL) study, 151 patients with AMI underwent excimer laser atherectomy. A SVG was the target vessel in 21%. A 95% device success rate, a 97% angiographic success rate, and a 91% overall procedural success rate were described. Maximal laser gain was achieved in lesions with extensive thrombus burden (p=0.03 versus small burden). The TIMI flow grade was increased significantly by the laser: 1.2±1.1 to 2.8±0.5 (p=0.001). Mean minimal luminal diameter (MLD) increased after laser from 0.5±0.5 to 1.6±0.5 mm (p=0. 001), achieving a final 2.7±0.6 mm diameter after stenting (p=0.001 versus baseline and versus post-laser). Laser decreased target stenosis from 83%±17% to 52%±15% (p=0.001). Six (4%) patients died, all of them presenting with cardiogenic shock. Complications included perforation (0.6%), dissection (5% major, 3% minor), acute vessel closure (0.6%), distal embolization (2%), and bleeding (3%). The study supported the concept of thrombus debulking by laser technology in the setting of AMI, obtaining maximal clot dissolution, a considerable increase in MLD and a good rate of restoration of anterograde TIMI flow.
In 2004 Ilkay et al evaluated the short-term results of percutaneous excimer laser angioplasty in a case series of AMI patients describing analogous procedural success rates. No randomized trial has compared - so far - laser technology to aspiration or mechanical thrombectomy.
ThromCat® mechanical thrombectomy catheter system
The ThromCat XT thrombus removal system is a single-use, disposable device intended for the removal of thrombus from native coronary and peripheral arteries. It uses the Heliflex™ technology to flush, macerate and extract thrombus with a helical stainless-steel wire, deployed in a 7 Fr braided jacketed catheter. The helix rotates at 90,000 rpm, creating a vacuum at the catheter tip for thrombus fragmentation and removal.
Structural point
- 150 cm working length
- 5 Fr tip crossing profile (distal end), 4.5 Fr catheter diameter (proximal end)
- 014” guidewire compatible
- Radiopaque distal end
- 10 cm rapid exchange lumen
The results of a European study to assess the device safety and performance during percutaneous coronary intervention were presented in 2009. The trial enrolled over 60 patients addressed to acute ore elective PCI, with intracoronary evidence of thrombotic content. Study results described over 90% of device success, achieving 70% thrombus removal by volume, improvements tin TIMI flow and myocardial blush scores, with 1.6% of patients requiring temporary pacing.
Distal protection devices
Distal Filters
Distal protection systems are generally placed distally in target vessel and aim at capturing thrombotic debris during PCI to prevent MVO. Filter-based devices are removed at the end of the procedure with embolic material inside. For occlusion-based devices (see next paragraph) target vessel aspiration needs to be performed before the protection is removed.
Distal filters (Filter embolic protection devices, EPD) incorporate or rail on a 0.014” guidewire with a terminal, self-expandable basket, which is pushed and unfolded from its delivery system after crossing the target lesion. During PCI, embolic fragments are captured while flowing, to be eventually withdrawn with the device at the end of the procedure. The core structure of filters is either a polyurethane membrane with laser-drilled holes or a nitinol mesh, which is mounted on a coronary wire that is used to cross the lesion and open the self-expandable device distally, by retrieving its folding sheath. The basket collapses for recapture it into a dedicated re-folding system, and is removed after PCI is completed together with embolized fragments.
The major advantage of a filter-based protection device is that antegrade perfusion and distal vessel visibility is preserved, since blood and solutes passage through the filter is granted .
Based on current evidence, the use of distal filter device is recommended during saphenous vein graft interventions but not for primary PCI with large thrombus burden.
FilterWire EZ™ EPS (Boston Scientific, Natick, MA, USA) (Figure 11 and Figure 12)
.png)
Figure 11
FilterWire EZ with basket deployed (Boston Scientific).
.png)
Figure 12
FilterWire EZ technical specifications (Boston Scientific).
The Boston Scientific FilterWire EZ System is an intravascular filtration device with 80 μm pores, which is pre-mounted on a 0.014” guidewire that is used to cross the lesion and deliver the filter distally in the target vessel inside a 6F-compatible EZ Delivery Sheath. A nitinol loop, with the filter attached, self-expands upon sheath’s withdrawal. A dedicated EZ Retrieval Sheath is used to re-fold and retrieve the filter with its content after PCI. The tip of the protection wire and the nitinol loop are radiopaque, to enable fluoroscopy visualization during positioning. The protection device is currently indicated for the treatment of saphenous vein bypass grafts and for carotid arteries. The diameter of the vessel at the site of filter loop placement should be between 2.25 mm and 5.5 mm for coronary saphenous grafts, with a minimal distal landing zone for wire tip accommodation. During PCI embolic material can obstruct the terminal basket, requiring its retrieval to restore blood flow.
Structural points
- PTFE coated wire (0.014”)
- 6 Fr guiding catheter-compatible system
- Minimum distal landing zone of 2.5 – 3.0 cm in length
- Target vessel diameter 2.25 – 5.5 mm
- Radiopaque tip and nitinol loop with 110 μm pore filter
- Reliable 360° wall apposition
Trials in myocardial infarction
PROMISE trial
After the BLAZE I and BLAZE II prospective multicenter registries provided the first indications for the use of FilterWire EZ System, the PROMISE trial enrolled 200 AMI patients with angiographic evidence of thrombotic occlusion, randomly assigned to receive or not filter-wire distal protection during angioplasty. The primary endpoint was the maximal adenosine-induced Doppler flow velocity in the infarct artery after reperfusion; among secondary endpoints infarct size was estimated by delayed gadolinium enhancement on MRI. The filter wire in PCI for the treatment of ACS did not improve blod flow nor reduced infarct size in comparison to standard PCI.
UPFLOW trial
The Use of Protective filter wire to improve FLOW in acute myocardial infarction (UPFLOW) trial was a multicentre, prospective clinical study, which randomized 100 STEMI patients with large thrombotic burden to FilterWire-assisted versus regular guidewires PCI. The FilterWire EZ was successfully delivered across the lesion in 84% of patients in the interventional group. Primary efficacy endpoints, including markers of epicardial (TIMI grade flow) and myocardial reperfusion (myocardial blush score and ST-segment elevation resolution), did not differ between the two groups. Authors concluded that the use of the FilterWire EZ as an adjunct to primary PCI did not improve angiographic or electrocardiographic measurements of reperfusion, compared to conventional PCI.
DEDICATION Trial
The DEDICATION Trial was the largest randomized, distal protection trial in a STEMI setting (n=626). Patients were treated with FilterWire or a SpiderFX protection device or without protection. There was no difference in terms of ST-segment resolution, maximum troponin-T or creatine kinase-MB levels, echocardiographic left ventricular wall motion index, or MACE among the two groups. Study results confirmed the futility of distal filers for the treatment of ACS.
Trials in saphenous vein grafts
EPD in SVG stenting
PCI on saphenous vein grafts with FilterWire EX was performed in 60 lesions in a Phase I experience, followed by a larger Phase II study on 248 lesions in 65 U.S. centres. In Phase I FilterWire was successfully delivered and deployed in 95.7% of cases. Postprocedural TIMI-3 flow was achieved in 94.7% of grafts. In-hospital MACE occurred in 18.8% of patients prior to hospital discharge. Most of them were periprocedural MI, possibly explained by geometric factors resulting in filter loop malapposition (i.e. large graft diameter), by very distal lesion location where the filter could not open properly or had to be placed in a native coronary vessel, leaving proximal branches unprotected, by balloon dilatations and/or stent implantations preliminary performed without distal protection or by heavily recaptured filters, whose retrieval provoked debris extrusion and embolization. In Phase II trial procedural outcomes were similar but angiographic complications were less frequent, with fewer episodes of no-reflow or macroscopic embolization. Moreover, 30-day cumulative MACE, with special regards to periprocedural MI, were significantly lower. Such results prompted the conduct of the following trials, specifically addressing clinical endpoints.
FIRE
A total of 651 patients undergoing percutaneous intervention on 682 vein graft lesions were prospectively randomized to filter-based distal protection with FilterWire EX vs. distal occlusion with GuardWire balloon system, a standard-of-care protection technique for SVG interventions with established good performances (see dedicated paragraph forward). Device success was similar (95.5% vs. 97.2% respectively; p=0.25), as post-procedural measures of epicardial flow and angiographic complications’ rate. Bailout GP IIb/IIIa inhibitors were never required in the FilterWire EX group (0% versus 1.5%, p=0.03). The primary endpoint (the composite incidence of death, myocardial infarction, or target vessel revascularization at 30 days) occurred in 9.9% of FilterWire EX patients and 11.6% of GuardWire patients (p for superiority=0.53, p for non-inferiority=0.0008). Consistently, FilterWire EX compared with distal protection with the GuardWire system resulted in similar rates of major adverse cardiac events at 30 days. Even in this trial, the rate of procedural complications significantly decreased from the preliminary to the final phase, showing non-inferiority of the filter-based strategy in comparison to vessel occlusion.
SpiderFX (eV3 Inc., Plymouth, MN, USA) (Figure 13 and Figure 14)
.png)
Figure 13
SpiderFX® embolic protection device (eV3) – deployed configuration.
.png)
Figure 14
SpiderFX® embolic protection device - retrieved configuration (eV3).
The SpiderFX™ Embolic Protection Device allows the use of any 0.014”- 0.018” coronary wire for lesion crossing, on which SpiderFX delivery catheter can then be advanced. The SpiderFX filter is deployed and recovered through a dual-ended and low-profile, 6F compatible delivery system. The capture filter is available in a range of sizes from 3 to 7 mm.
Structural points
- Atraumatic lesion crossing on any 0.014” or 0.018” guidewire
- 6 Fr guide-compatible
- Rapid Exchange (RX) delivery catheter with low (3.2 Fr) crossing profile
- Enhanced visualization of the radiopaque filter mouth
Trials in AMI
PREMIAR
The Protection of Distal Embolization in High-Risk Patients with Acute ST-Segment Elevation Myocardial Infarction Trial (PREMIAR) was a prospective, randomized, controlled study designed to evaluate the role of filter-based distal protection during PCI in patients with acute STEMI at high risk of embolic events (including only baseline TIMI grade 0 to 2 flow). The primary endpoint was ST-segment resolution. The rate of complete ST-segment resolution at 60 minutes was similar (61.2% vs. 60.3%, respectively, p=0.85). As secondary endpoints, angiographic myocardial blush (67% vs. 70.7%, p=0.73), in-hospital ejection fraction (47.4±9.9% vs. 45.3±7.3%, p=0.29) and a combined clinical endpoint of death, heart failure, or reinfarction at 6 months (14.3% vs. 15.7%, p=0.81) did not differ in the two groups. Once more, authors concluded that the use of filter-based distal protection did not translate into an improvement of myocardial reperfusion, left ventricular performance, or clinical outcomes in patients with AMI referred for PCI.
Trials in SVG
SPIDER
In the Saphenous Vein Graft Protection In a Distal Embolic Protection Randomized Trial, 732 patients with SVG lesions in 80 clinical sites were randomised to Spider/SpideRX (n=375) or GuardWire (24%)/FilterWire (76%) (n=357) protected PCI for a non-inferiority analysis, with a primary endpoint of MACE at 30 days (death, MI, TVR, urgent CABG). Non-inferiority was demonstrated (p=0.012); non-significant differences were found for both safety and efficacy secondary endpoints.
DEDICATION Trial
See above.
CardioShield® (Abbott Laboratories, Abbott Park, IL, USA)
The CardioShield Bare Wire Myocardial Protection System is available as either an over-the-wire (OTW) or a rapid exchange (RX) device. The filtration element, consisting of a polyurethane outer membrane with supporting nitinol arms, is available in 3.0 mm, 4.0 mm, 5.0 mm and 6.0 mm nominal diameters. The delivery catheter is advanced with the filter wire and deployed by retracting its outer sheath. PCI is then performed over the same wire, after which the filter is retrieved.
Trial
CAPTIVE
CardioShield Application Protects During Transluminal Intervention of Vein Grafts by Reducing Emboli (CAPTIVE) was a prospective, multicentre randomized clinical trial which evaluated this third-generation distal protection device vs. GuardWire in 652 patients undergoing treatment of SVG disease, with MACE as a primary endpoint. This study assessed the superiority of the CardioShield vs. no protection and the non-inferiority between CardioShield and GuardWire devices, consistently with the results obtained by the other filter-based protection devices.
TRAP (Microvena, White Bear Lake, MN, USA)
The TRAP Vascular Filtration System is a nitinol mesh fixed at the distal end of a polytetrafluoroethylene-coated 0.014” guidewire with a flexible tip. The filter is available in 2.5 to 7 mm diameter sizes and is delivered in a 2.9 Fr delivery catheter. The guidewire and the filter are covered with a heparin coating, allowing 60 minutes' persistence inside the vessel without any thrombin or fibrin adhesion.
Trial
TRAP
The purpose of this prospective, multicentre trial was to evaluate the safety and effectiveness of the TRAP Vascular Filtration System (VFS) to reduce embolic complications during stenting of diseased SVGs. Patients with SVG lesions were randomly assigned to undergo stenting with or without the TRAP device. The primary study endpoint, major adverse cardiac events at 30 days, occurred in 17.3% of control patients and 12.7% of patients treated with the TRAP device (p=0.24). The study was terminated prematurely.
TRAP is currently used mainly as a cerebral protection filter during carotid interventions.
Interceptor® PLUS (Figure 15 and Figure 16)
.png)
Figure 15
Interceptor PLUS system (Medtronic).
.png)
Figure 16
Interceptor PLUS system, benchtop model (Medtronic).
Interceptor PLUS is a braided nitinol filter mounted near the distal tip of a steerable 0.014 inch. guidewire, which extends 3.5 cm beyond the filter.
Trial
AMETHYST
The Interceptor PLUS Coronary Filter System was compared with approved embolic protection devices (e.g., GuardWire, Medtronic Vascular/FilterWire EZ) during PCI of SVGs. In this multicentre, randomized non-inferiority trial, 797 patients undergoing PCI with stenting of SVG stenoses (de novo or restenotic), with reference vessel diameter 2.5 mm to 5.25 mm, were randomly assigned 2:1 to either the Interceptor PLUS (n=533) or control distal protection devices (GuardWire [n=191], FilterWire EZ [n=73]). The trial demonstrated the non-inferiority of this embolic protection device in terms of composite occurrence of death, myocardial infarction or urgent repeated revascularizations up to 30 days (8% vs. 7.3% for Interceptor and controls, respectively). Key secondary endpoints for device and procedural success were also similar.
Occlusion-based systems
In contrast to filter embolic protection devices, balloon occlusion of target vessel implies cessation of antegrade flow during index PCI. To achieve distal occlusion, the balloon is mounted on a wire that allows distal device positioning and, during balloon inflation, PCI performance. Debris are removed at the end of the procedure with an aspiration catheter, prior to antegrade flow restoration. The alternative approach is based on proximal occlusion, arresting blood in culprit vessel proximally to the target lesion. Once more, aspiration of the blood column is performed after PCI, prior to antegrade flow restoration.
Distal and proximal balloon occlusive devices´ goal is a complete retrieval of debris suspended in the blood column at the time of intervention. Clear disadvantages of the occlusive approach are the overt ischemic potential and the poor visualization of the lesion during the procedure.
Distal Occlusion
PercuSurge GuardWire (Medtronic Vascular, Santa Rosa, CA, USA) (Figure 17 and Figure 18)
.png)
Figure 17
PercuSurge GuardWire (Medtronic) catheter system.
.png)
Figure 18
PercuSurge GuardWire - deployed configuration (Medtronic).
The GuardWire Plus (Medtronic Vascular, Santa Rosa, CA, USA) consists of a 0.014” guidewire, distally attached to an elastomeric balloon interrupting antegrade blood flow while inflated through an inflation lumen , . The elastomeric polyurethane balloon can be inflated from 3 mm to 6 mm. Intervention is then performed over the wire, after which liberated debris suspended within the stagnant blood column are aspirated through a 5 Fr monorail Export catheter. The correct elastomeric balloon position is as close to the lesion as possible, to minimize exposure of unprotected side branches to embolic debris. The following trial show a benefit in SVG PCI but not in AMI patients, for whom distal occlusion is currently not recommended.
Trials in STEMI
EMERALD
The Enhanced Myocardial Efficacy and Recovery by Aspiration of Liberated Debris (EMERALD) trial was a prospective, randomized, multicentre trial, testing distal balloon protection during primary or rescue PCI in STEMI patients presenting within 6 hours of symptom onset. Co-primary efficacy endpoints were ST-segment resolution (STR) 30 minutes after PCI and infarct size, measured by technetium 99mTc-sestamibi imaging between days 5 and 14. Secondary endpoints included major adverse cardiac events.
Complete STR was achieved in a similar proportion of patients, with or without distal protection (63.3% vs. 61.9%; absolute difference, 1.4% [p=0.78]); left ventricular infarct size was similar in both groups (median, 12.0% [n=229] vs. 9.5% [n=208], respectively; p=0.15). Major adverse cardiac events at 6 months occurred with similar frequency in the distal protection and control groups (10.0% vs. 11.0%, respectively; p=0.66). The trial showed no benefit of distal occlusion in AMI patients.
ASPARAGUS
From February 2002 to July 2003, a total of 341 AMI patients at 22 institutions in Japan were enrolled within 12 hours from symptoms onset in this multicentre, prospective, randomized trial to compare culprit lesion stenting with and without GuardWire. A significant difference was found between the GuardWire Plus and control groups with respect to the total incidence of distal embolization, indicating a possible benefit of GuardWire Plus in terms of angiographical indices of myocardial perfusion. However, there was no evidence that the GuardWire Plus improved ST-segment resolution, infarct size or myocardial blush score, primary endpoints of the study.
MICADO
MICADO was a prospective, multicentre trial on acute AMI patients randomized to PCI with or without GuardWire distal protection. The primary endpoints were TIMI flow and incidence of no-reflow. Secondary endpoints were major cardiac events (MACE) at 6-month follow-up. The incidence of no-reflow was similar (4% vs. 3%). TIMI 3 was more frequent in the GuardWire group, but without statistical significance (58% vs. 44%; p=0.054). No difference was shown in the occurrence of MACE.
Trials in SVG
SAFER
The primary objective of the Saphenous vein graft Angioplasty Free of Emboli Randomized (SAFER) trial was to compare 30-day clinical outcomes after saphenous vein graft stenting with and without GuardWire distal protection. The primary endpoint of the study was MACE rate at 30 days, and the trial enrolled 801 patients in 47 sites. There was a significant reduction in the primary endpoint (9.6% for GuardWire patients versus 16.5% for control patients; p=0.004), predominantly led by a reduction of myocardial infarctions. In addition, rates of TIMI grade 3 flow were higher for the GuardWire arm (98%) compared with the control arm (95%; p=0.04), and the incidence of clinically evident no-reflow was relevantly reduced (3% versus 9%; p=0.001). Favorable results of this trial significantly contributed to the establishment of the GuardWire strategy as a standard protection technique for SVG PCI, and explain why it was used in the control arm of several subsequent clinical trials aiming at showing non-inferiority of filter-based systems in the same interventional context (see above).
TriActiv system (Kensey Nash Corporation, Exton, PA, USA)
The TriActiv FX™ system has a compliant CO2-filled distal balloon integrated on a 0.014” guidewire and coupled with an active flush and extraction device. The device is inflated with CO2 (through the ShieldWire Inflator) during contrast injection, so that the contrast dye is trapped in the vessel to warrant lesion visibility during PCI. After angioplasty has been performed, a monorail flush catheter (FX™ catheter) is advanced over the integrated balloon guidewire to remove the contrast column and the floating debris.
Structural points
- Rapid inflation and deflation of the balloon with CO2
- Shorter distal “landing zone” required
- Automated active flushing system working on balloon surface, vessel wall and stent struts, to extract adherent debris.
Trials
PRIDE
The Protection During Saphenous Vein Graft Intervention to Prevent Distal Embolization (PRIDE) study compared outcomes with the TriActiv System vs. other protection devices (GuardWire System or Filterwire EX in the control group).
The incidence of major adverse cardiac events at 30 days was 11.2% for the TriActiv group and 10.1% for the control group (relative risk = 1.1%; 95% confidence interval 0.67 to 1.76; p=0.65; p=0.02 for non-inferiority). Patients randomized to the TriActiv System had more hemorrhagic complications (10.9% vs. 5.4%; p=0.01).
Proximal occlusion
Proximal occlusion is based on the same principle of suspending anterograde blood flow during PCI and then aspirating the stagnant column of blood and embolic debris before flow restoration, with the difference that occlusion of the target vessel is proximal. Since the device does not cross the lesion, the risk of iatrogenic embolization is reduced.
Kerberos Embolic Protection Technology Rinspiration (KEPT) system (Kerberos PS, Sunnyvale, CA, USA):
The Kerberos Embolic Protection Technology System is designed to provide proximal balloon occlusion of the target SVG during PCI. This 8 Fr guiding catheter incorporates a compliant occluding balloon at its distal tip, inflated through a dedicated second lumen after SVG cannulation. Rinse is an over-the-wire, 4 Fr catheter with multiple distal perforations located between two distal marker bands, advanced over a standard 0.014’’ wire beyond target lesion for blood aspiration. Rinspirator is a hand-activated device allowing for simultaneous irrigation and aspiration of the graft through the Rinse catheter.
Procedure requires a 8.5-9 Fr femoral arterial sheath to cannulate the graft. The balloon at the tip is inflated to approximately 2 atm, to achieve proximal occlusion. A standard angioplasty wire is positioned distal to the lesion with the aid of previously determined fluoroscopic landmarks or with a small injection of contrast, to avoid wire-induced embolization. Balloon dilatation and/or stent implantation is performed. Then the rinse catheter is introduced over the coronary guidewire beyond the lesion. The Rinspirator is manually activated at the rate of 1 squeeze/sec and retrieved in a distal-to-proximal direction. The occlusion balloon is deflated after PCI, restoring coronary perfusion .
Procedure feasibility was shown in small case series, but no trials are currently available.
PROXIS (ST. Jude Medical, St. Paul, MN, USA) (Figure 18 , Figure 19 and Figure 20)
.png)
Figure 19
Proxis system (St. Jude Medical).
.png)
Figure 20
Proxis system deployed stopping antegrade blood flow during protected PCI (St. Jude Medical).
The Proxis system incorporates a sealing balloon that is deployed proximal to the target stenosis, to create a stagnant column of blood in which intervention is performed. The stent is delivered through the Proxis system, flow is reversed to aspirate debris and the balloon is then deflated, to restore blood flow.
A proximal landing zone of 12 mm is needed, so ostial lesions are not suitable for protected treatment with this device.
Trials
PROXIMAL trial
A total of 594 patients undergoing stenting of 639 SVGs were prospectively randomized, using a non-inferiority design, to compare proximal protection with Proxis to distal protection (whenever possible). The primary composite endpoint of death, myocardial infarction, or target vessel revascularization rate at 30 days was 10.0% among controls and 9.2% in the experimental arm (p for non-inferiority=0.0061). In device-specific analysis, the composite endpoint occurred in 11.7% of distal protection patients and 7.1% of proximal protection patients (difference=4.6% [95% CI: 9.6% to 0.3%]; p for superiority=0.10, p for non-inferiority=0.001), suggesting a favorable profile.
PREPARE
The purpose of the PRoximal Embolic Protection in Acute myocardial infarction and Resolution of ST-Elevation study this study was to evaluate the effectiveness of Proxis Embolic Protection System in 248 STEMI patients, randomized to primary PCI with or without proximal occlusion. The primary endpoint was the occurrence of complete (≥70%) ST-segment resolution (STR) at 60 minutes, without any significant difference (80% vs. 72%, p=0.14) between the interventional and the control group. Major adverse cardiac and cerebral events at 30 days occurred with similar frequency in both groups, showing no benefit.
Proximal balloon occlusion flow reversal system
Beyond thrombectomy and distal protection: a glance on alternative strategies to prevent or manage coronary embolization in ACS
If protection devices have achieved good results for the management of embolic events in SVG-PCI, coping with large thrombus burden in acute coronary syndromes is still an unsolved challenge. Thrombectomy techniques have been widely implemented in clinical practice in the past decades, but latest studies have proved poor clinical benefits. Such an unmet medical need has brought to theorize alternative strategies to prevent or manage embolic-based microvascular obstruction, which are nowadays being proposed and tested in clinical trials .
Modified stent platforms to prevent distal thrombotic embolization
- MGuard™ Coronary Stent and Embolic Protection Device (InspireMD Inc., Tel Aviv, Israel) is the combination of a bare metal coronary stent surrounded by an ultra-thin, porous polymeric sleeve, engineered to trap thrombus fragments on an under-struts layer and allow stent endothelization. Concerns about side branch occlusion, device deliverability and in-stent restenosis have limited its use, especially in the era of drug-eluting stents .
- STENTYS are self-expanding stent platforms designed to address the challenges of coronary arteries with variable diameter across the lesion or with large side branches. The device is manufactured in nitinol, to achieve vessel conformability and continuous apposition over time along the arterial wall. This can avoid stent post-expansion, as a potential source of emboli in the context of thrombotic lesions. Moreover, self-expansion is supposed to continue with thrombus progressive fragmentation, thus preventing delayed malapposition. Notwithstanding this rationale, a net benefit for this stent technology in comparison to standard DES has not been demonstrated .
Devices to achieve thrombus fragmentation
- Sonothrombolysys relies on the capability of ultrasounds to achieve thrombus fragmentation. After an ultrasound-enhancing intravenous agent has been administered, standard ultrasound transducers are used to deliver high mechanical index (HMI) impulses (ideally while the patient is transferred to the closest PCI center). US erogation aims at inducing thrombus cavitation and anticipate culprit vessel reperfusion while the patient is being transferred to the closest PCI-capable center. In a randomized clinical trial, HMI-treated patients showed reduced infarct size and higher left ventricular ejection fraction in comparison to primary PCI alone . Further evidences are required to assess the potential impact of this technology on clinical outcomes.
Devices for local drug delivery
Beside invasive approaches, the management of thrombus burden in acute coronary syndromes largely relies on antithrombotic pharmacological agents, which have paramount importance in achieving and maintaining coronary patency during and after PCI. A combination of antiplatelet and parenteral anticoagulants is nowadays part of standard clinical practice in the acute phase of ACS, and medications are tailored case by case to address negotiate ischemic and bleeding risks. Intracoronary low-dose infusion of antithrombotic drugs aims, as a strategy, at maximizing their antithrombotic effect in the culprit district while reducing the risk of bleedings, which is typically dose-dependent and related to their systemic distribution. The following devices have been accordingly engineered with this purpose:
- The ClearWay™ RX device (Atrium Medical Corp., Hudson, NH, USA) was designed to selectively deliver intracoronary drugs in the target vessel, increasing their local bioavailability and uptake while minimizing their systemic distribution through a dedicated balloon that engages the vessel wall, occludes antegrade blood flow and allows a controlled pharmacological infusion from the tip. The COCTAIL II randomized clinical trial, however, didn´t find any advantage in terms of residual thrombus burden in comparison to a standard intracoronary administration of the same GpIIb-IIIa inhibitor (abciximab) .
- The CorFlow Controlled Flow Infusion System aims at a local drug delivery through a dedicated balloon catheter (CoFI™, CorFlow Therapeutics AG), which is coupled with a coronary pressure wire. The balloon is advanced on the wire to reach the target lesion after PCI has been successfully completed. While the balloon is inflated, distal pressure response to a controlled antegrade infusion of saline solutions provides an estimation of microvascular resistances. Drugs can then be delivered through the balloon tip and resistances estimation can be repeated to verify their effect. The device is currently under investigation in the first-in-human MOCA I Trial, with the aim of defining procedural safety and feasibility and of exploring a possible correlation between resistances´ measurement and MVO detection at contrast MRI (NCT03654573).
Devices to minimize microvascular injury
- PiCSO technology induces high pressures in venous coronary circulation during primary PCI through an intermittent balloon occlusion of coronary sinus. High venous pressures aim at recruiting collateral circles, re-directing blood from remote to ischemic myocardium. During balloon deflation phases thrombotic debris, catabolites and oxidative products of myocardial necrosis are washed out from the infarct area. Preliminary studies have shown the safety and feasibility of the procedure in ACS patients, improved microvascular functionality at 24 h and a reduced infarct size at four and six months. PICSO-AMI I is a multicenter trial, currently randomizing STEMI patients to PiCSO + primary PCI vs. PCI alone and powered to assess the ability of this technology to reduce infarct size at 5 days , .
An overview on recommendations and current use of thrombectomy, proximal and distal protection
Acute coronary Syndromes
The management of large thrombotic burden in the context of acute coronary syndromes has aroused an abiding concern, as embolic phenomena are invariably linked to microvascular injury and unsuccessful reperfusion.
Distal (filter or occlusion-based) protection devices have found no room in this clinical context for an overt lack of benefit, that can be explained by several shortcomings. First, they could themselves promote thrombus embolization during lesion crossing, while the final removal of debris after PCI can be partial. Moreover, predilatation of the lesion is often required to be crossed with the balloon or the filter, and even after positioning embolization in uncovered side branches can always occur. Similarly, proximal occlusion and mechanical thrombectomy have never shown clinical benefits supporting their use.
Manual aspiration techniques were initially welcomed enthusiastically - after TAPAS and EXPIRA trials were published – and were diffusely adopted for the management of thrombus burden in acute coronary syndromes. Subsequent negative performances in larger RCTs, powered to include strong clinical outcomes among primary endpoints (INFUSE-AMI , TASTE and TOTAL ), drove the restriction in indications provided both by American , and European Guidelines, downgrading recommendations for routine aspiration thrombectomy in AMI to class III-A and IIb-A, respectively.
Study-specific aspects of these trials have been widely discussed and need some considerations. In TASTE trial more than two thirds of patients had a small thrombotic burden. The effect of thrombectomy in this large population could rationally be limited, but subgroup analysis did not suggest any interference of thrombotic burden on aspiration outcomes. The study was not considered conclusive but was followed by TOTAL, which gave a start to the decline of manual thrombectomy for the treatment of STEMI patients. A lower rate of incomplete ST-segment resolution and of embolic events in patients treated with thrombectomy prior to standard PCI were observed, but they did not translate into any clinical benefit. High cross-over rates (230 patients from thrombectomy to PCI-alone, 355 patients assigned to PCI-alone required bailout thrombectomy) didn´t apparently impact trial results, which were consistent in the intention-to-treat and in per-protocol analyses. Not secondarily, an increased rate of stroke in the interventional arm led to remarkable safety concerns, although the study was not adequately powered for this event.
After TOTAL, a meta-analysis of 17 RCT including more than 20,000 subjects further confirmed no benefit for routine thrombus aspiration with respect to death, reinfarction or stent thrombosis, with a small (but not statistically significant) increase in the risk of stroke . For these reasons, this RCT is nowadays considered a cornerstone in the history of manual thrombectomy, which is consistently discouraged as a routine approach in STEMI patients.
Nevertheless, thrombus aspiration is still performed in around 15% of primary PCI when large thrombus burden must be tackled, since alternative, safer and more effective protecting strategies are not available. Current research is widely focusing on new thrombectomy techniques and on alternative strategies to tackle large thrombus burden and prevent microvascular injury in ACS. New effective and safe devices are needed, and could be on the shelf of interventional cardiologists in the next few years.
Experimental insights on these state-of-the-art techniques are still required and need to be strongly encouraged, to address this high-impact, unmet medical need.
Saphenous vein graft interventions
Unlike what happened in the acute setting of myocardial infarction, the use of protection devices can represent a game changer in SVG PCI. The SAFER was halted early for a significant benefit associated with the use of GuardWire on the primary composite endpoint of death, MI, emergency bypass, or target lesion revascularization at 30 days, mainly driven by a substantial reduction in post-procedural MI. Comparative studies among different types of EPD followed, showing non-inferiority of the filter-based approach with respect to MACE.
According to this consistent bulk of evidence, the use of protection devices in SVG interventions received a class I (B) recommendation in ESC and ACC)/AHA , guidelines, but it is still far from being part of routine clinical practice and requires efforts for further implementation.
Personal perspective - Marco Valgimigli
The pathophysiological rationale for the use of thrombectomy catheters and/or protection devices, to limit distal embolization during PCI, is unquestionable.
It is however clear that neither of these strategies achieved the expected results. The history of the two strategies differ.
Protection devices is a “mature” technology and their use is confined to SVG intervention. Yet, they are underutilized in SVG procedures, despite supportive guideline recommendations.
The role of thrombectomy during primary PCI has been recently revisited based on most recent evidence from randomized trials which failed to show its effectiveness.
Yet, thrombectomy remains a highly desirable goal in contemporary PCI, especially in ACS patients with large thrombus burden and recent studies have been investigating promising new approaches to tackle this old limitation of so called “conventional” PCI.