Evangelos Giannitsis, Christian W. Hamm, Holger M. Nef, Hugo A. Katus
Updated on May 14, 2022
CAD – Coronary artery disease
CIN – Contrast induced nephropathy
CKD – Chronic kidney disease
iFR – Instantaneous wave-free ratio
IVUS – Intravascular ultrasound
PCI – Percutaneous coronary intervention
ULCPCI – Ultra-low contrast percutaneous coronary intervention
Over its entire history, percutaneous coronary intervention (PCI) has been linked to contrast opacification of coronary vessels through the use of iodinated contrast. As a consequence, it has been tacitly assumed that the liberal use of contrast media when undertaking percutaneous coronary intervention (PCI) is needed to achieve an optimal procedure. Yet, in specific patient subsets the administration of iodinated contrast during PCI can have detrimental effects, secondary to fluid overload, organ toxicity of contrast or to hydraulic injury caused by injections (e.g., coronary dissections). While chronic kidney disease (CKD) has been customarily identified as a scenario for reduced contrast administration during PCI, to decrease the risk of acute kidney injury (AKI), there are many other scenarios in which an ultra-low contrast (ULC) PCI approach may be helpful, particularly in patients with complex or high-risk clinical or anatomical profiles. Reaching the upper limit of a safe dose of iodinated contrast may become a limiting factor in completing complex PCI. When addressing complex PCI and/or high-risk patients, reducing the amount of contrast needed for completing revascularisation is a crucial step to minimise complications and achieve successful results. In this regard, various contemporary PCI tools and manoeuvres may be adopted to reduce the amount of contrast media and fluids administered during the procedure. This compendium of techniques is intended to extend the benefits of PCI to high-risk scenarios and populations that are excluded from revascularisation, to reduce operator dependence on contrast for completing high-quality procedures and to avoid complications such as contrast-induced nephropathy (CIN), haemodynamic instability due to fluid overload, iatrogenic coronary dissections, etc.
So far, much of the attention has been put on the prevention of CIN, a complication that can affect up to 15-20% of high-risk patients undergoing PCI , and is associated with significant morbidity and mortality , . However, the adoption of strategies that limit contrast infusion during PCI should not be regarded solely as a preventive strategy of CIN but rather as a broader approach to complex PCI in high-risk patients.
This chapter summarises the concept of ultra-low contrast percutaneous coronary intervention (ULCPCI) and the different procedural strategies aimed at reducing the use of contrast during PCI. For a more detailed description of CIN and preprocedural strategies for its prevention, we refer the reader to the dedicated chapter in this textbook.
The reluctance of operators to perform invasive procedures in patients with CKD is known as renalism . This phenomenon leads to an undertreatment of coronary artery disease (CAD), both in terms of quantity of patients referred for PCI and the quality of the interventions performed. In addition, it is not only limited to patients with renal disease but has also been observed in complex cases where concerns regarding contrast media and volume administration might hamper the access and quality of PCI.
ULCPCI is defined as an intervention where the ratio between the volume of contrast administered and the estimated glomerular filtration rate (eGFR) is less than 1 . This quotient between contrast and creatinine clearance has been shown to be independently associated with CIN and can be used as a straightforward parameter to guide the desired volume of contrast which can be administered in ULCPCI procedures . Whilst the ULCPCI definition only considers the volume administered, its philosophy integrates several important parameters, such as operator experience, technological advances, and the intensive use of intracoronary imaging and physiology, in order to achieve high-quality results.
The objectives of ULCPCI are not only to minimise the risk of CIN but also to allow the completion of high-quality procedures in high-risk patients, thereby reducing the dependence on contrast injections to achieve success (Figure 1). This issue is of significant importance as complex coronary artery disease, renal impairment, and significant comorbidities often present together. Therefore, the adoption of ultra-low contrast methods during PCI could have several advantages: 1) it allows for the treatment of patients that could otherwise not have undergone the indicated procedure; 2) it reduces the dependence on contrast during PCI; and 3) it fosters the adoption of other techniques that holistically increase the quality of revascularisation, such as intracoronary imaging and physiology.
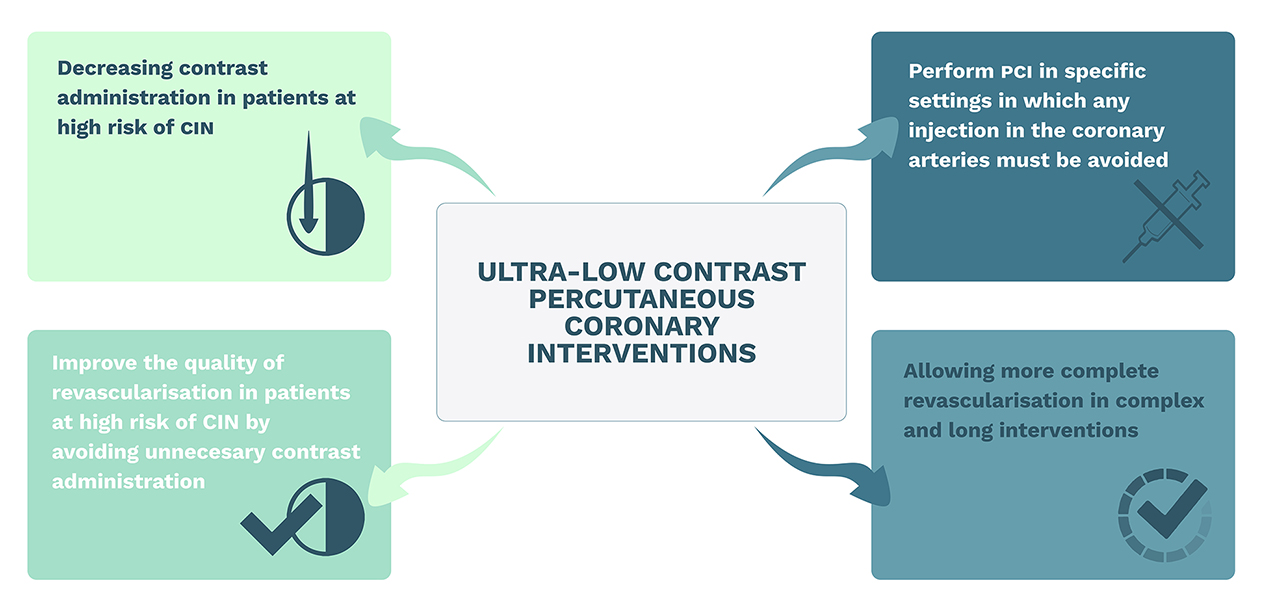
Main objectives of ultra-low contrast percutaneous coronary interventions.
CIN: contrast-induced nephropathy; PCI: percutaneous coronary intervention
The integration of ULCPCI strategies is useful in several specific clinical scenarios; however, there are some scenarios in which maximum benefit can be expected:
A broader list of the clinical scenarios that can benefit from ultra-low contrast coronary interventions is shown in Table 1. As operators become proficient in contrast-sparing practices, it is logical that its implementation will also extend to other settings in everyday practice.
Table 1. Clinical contexts for ULCPCI
| Clinical contexts for ULCPCI | |
|---|---|
| Chronic kidney disease | Very severe LV dysfunction |
| Prior acute kidney injury | Severe contrast allergy |
| Renal transplant | Severe thyroid dysfunction |
| Concomitant contrast-based tests (CTCA…) | Anticipated long PCI procedures (multivessel disease, CTO…) |
| Shock/out-of-hospital cardiac arrest | Iatrogenic coronary dissections |
| Emergency PCI with unknown eGFR | Spontaneous coronary dissections |
| Patient frailty | CTO treated with CART /ADR |
| ADR: antegrade dissection re-entry; CART: controlled antegrade and retrograde tracking; CTCA: computed tomography coronary angiogram; CTO: chronic total occlusion; eGFR: estimated glomerular filtration rate; LV: left ventricular; PCI: percutaneous coronary intervention; ULCPCI: ultra-low contrast PCI | |
The appropriate selection of vascular access is important when planning ULCPCI. Observational data suggest that the use of transradial access is associated with a lower incidence of CIN when compared to the femoral artery , , data that have been corroborated by the prospective MATRIX trial. In this study, patients with acute coronary syndromes were randomised to either transradial or transfemoral access, with transfemoral access demonstrating an increased rate of CIN (17.4% vs 15.4% with transradial access; p=0.018). Interestingly, the protective effect of radial access was more pronounced in patients with clinical risk factors for CIN, such as reduced eGFR and a higher Killip class .
The relationship between vascular access and CIN can be influenced by various causes, notably the lower rates of bleeding associated with a transradial approach: major bleeding and subsequent haemodynamic instability may impair renal perfusion and increase the concentration of contrast media in the kidney parenchyma, enhancing its nephrotoxic effects. In addition, the manipulation of catheters in the abdominal aorta in transfemoral access may play a role in the occurrence of CIN. For these reasons, radial access, when feasible, should be the primary option when planning an ULCPCI procedure.
The appropriate selection of the guiding catheter curve and size is an important initial step towards the minimisation of contrast media. Smaller catheters (5-6 Fr) without side holes will improve vessel opacification with each injection. In all cases, correct coaxial engagement of the coronary ostia is mandatory. In a high proportion of cases, this engagement can be determined using fluoroscopy alone, especially by experienced operators. However, sometimes it is not evident with fluoroscopy, and correct positioning of the catheter is difficult to assess. Coronary calcification seen in fluoroscopy can be used as an anatomical landmark for the localisation of the ostia. By increasing the frame rate, identification of the calcified silhouette can be improved. When the catheter is correctly engaged, the injection of manual boluses of heparinised saline produces changes in ventricular repolarisation, which can be used to confirm catheter engagement without the need for contrast puffs (Figure 2). The administration of 10-20 ml of saline will produce dynamic changes in T-wave amplitude (inversion or increased amplitude) and/or ST-segment depression or elevation if the catheter is correctly engaged. In addition to this, the advancement of an intracoronary workhorse wire that runs into a coronary vessel can also confirm the correct position.
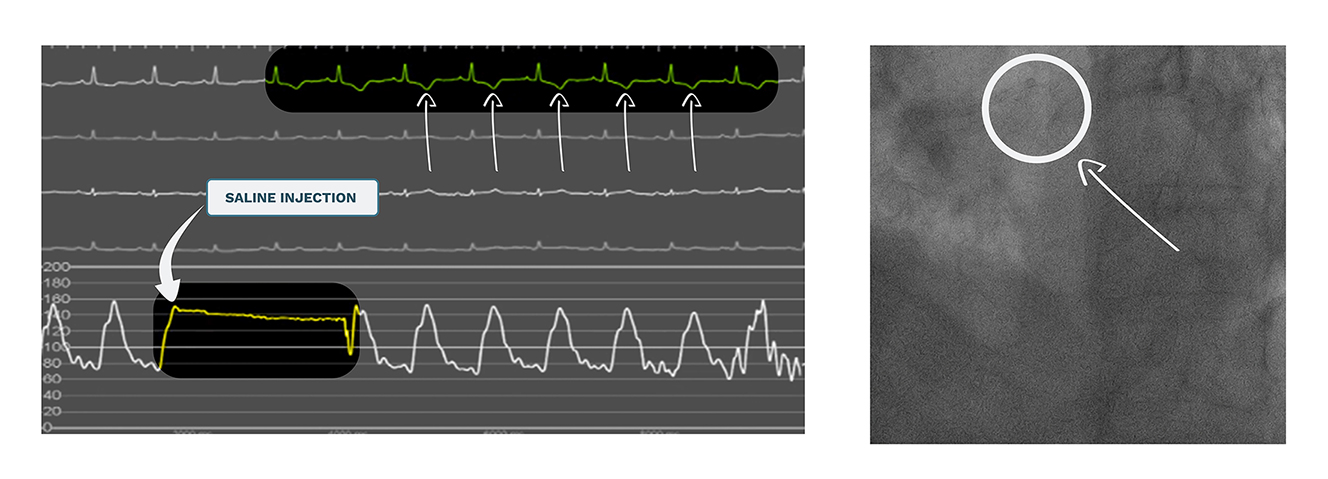
Ostium engagement and ECG changes due to saline injection.
Panel A (left) shows ECG monitoring before and after the infusion of a bolus of saline (5-7 ml approx.). Immediately after the injection, negative T-waves can be seen in the ECG, confirming the adequate engagement of the guiding catheter into the coronary ostium (panel B, right).
ECG: electrocardiogram
Once the guiding catheter is in place, the use of guide extension catheters allows for deeper, subselective target vessel cannulation. A careful low-volume injection of contrast through the guide extension lumen can significantly reduce the volume of dye needed for the opacification of a given vessel .
The volume of contrast media is a major determinant for the risk of CIN. The use of diluted contrast (with at least a 1:1 ratio using normal saline) is a simple measure that can greatly decrease the amount of contrast administered to the patient while maintaining sufficient opacity of the coronary arteries. Some centres with established ULCPCI protocols have introduced diluted contrast as the standard approach for these interventions (Figure 3).
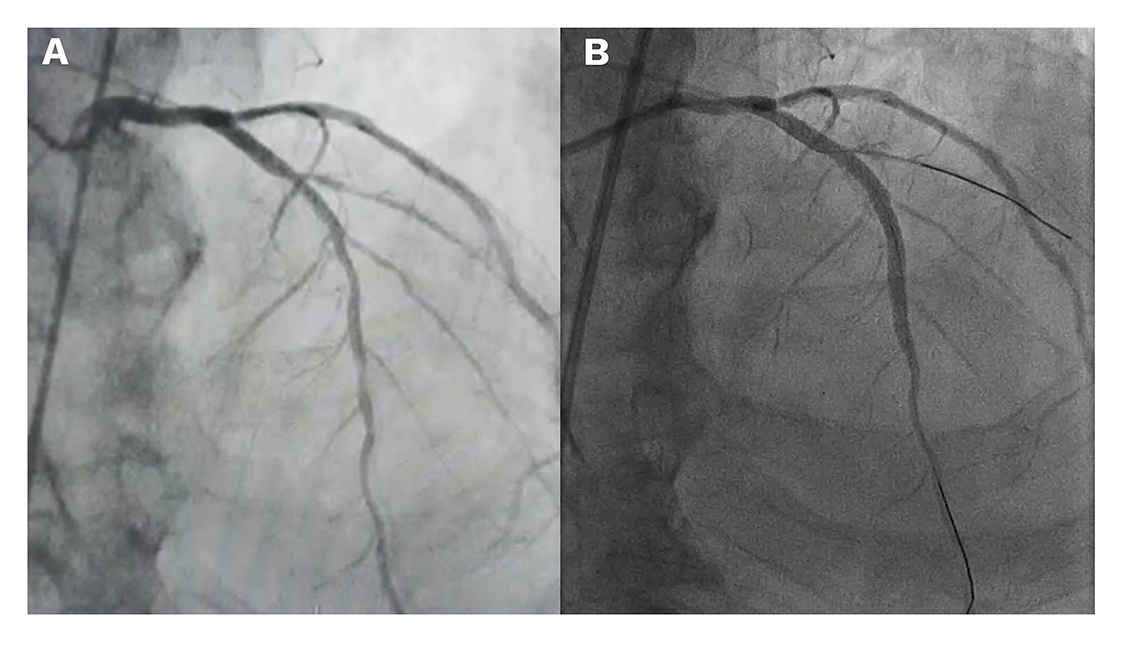
Coronary angiograms obtained with normal contrast and diluted contrast.
The left image (A) shows a conventional angiogram using 6 ml of contrast and 15 frames per second. The image on the right (B) shows an angiography of the same patient undergoing a scheduled ULCPCI procedure using 6 ml of diluted contrast (1:1 ratio with normal saline) and higher frame rate (30 frames per second).
ULCPCI: ultra-low contrast percutaneous coronary intervention
Coronary calcification is more common in patients with CKD and in high-risk complex procedures . Similar to what has been previously described, calcification of the coronary tree can assist the operator with recognition of the vessel’s silhouette without the need for contrast injections. The visualisation of coronary calcium can be enhanced by increasing the frame rate and may be sufficient for wire navigation and stent placement.
The use of patients previous coronary angiograms is another simple step that can aid operators to navigate through vessels, without the need for further injections. These diagnostic images can be displayed on a separate screen during the PCI procedure. The use of intracoronary wires can aid in marking the important side branches that should be taken into consideration during PCI of the target vessel. This ‘skeleton’ of wires serves as a landmark for balloon dilatation and stent placement. By creating knuckles at the tip of the wire, and using wires without a hydrophilic coating, the risk of distal perforation is diminished.
When no previous coronary angiograms are available, various strategies can be used to maximise the information gathered from contrast injections. Some laboratories can perform biplane coronary angiography, but this technology is not widely available. The recently developed Dynamic Coronary Roadmap (DCR; Philips Medical Systems, the Netherlands) is software that displays a dynamic template of the coronary tree on top of a live fluoroscopic image. This ‘map’ is automatically generated from an angiogram and can aid the navigation of wires and material during PCI, avoiding the need for contrast puffs to determine the position within the coronary vessels (Video 1). In a feasibility study by Piayda et al comprising 36 patients undergoing PCI, DCR proved to be accurate and safe, without any complications related to the software . In a subsequent study comparing DCR versus standard practice, the use of this technology led to a significant reduction in radiation exposure (11.4 vs 17.5 minutes of fluoroscopy time; p<0.01) and contrast media administration (118.8 vs 158.7 ml; p<0.01) . In order to confirm these findings, the results of the ongoing prospective multicentre randomised Dynamic Coronary Roadmap for Contrast Reduction (DCR4Contrast) clinical trial are awaited (ClinicalTrials.gov: NCT04085614).
Use of dynamic coronary roadmap (DCR) for wire navigation.
This is an example of how DCR displays a template of the coronary vessels generated from a previous angiogram. The overlapping image can help the wiring of the target vessel without the need for further contrast injections.
Traditionally, the significance of a given coronary stenosis and decisions pertaining to revascularisation, have been established by the degree of visually estimated stenosis of the coronary angiogram. However, with the development of pressure wires, the use of intracoronary physiology to guide revascularisation has been demonstrated to be superior than angiography alone , . More recently, non-hyperaemic indices have also been shown to be comparable to fractional flow reserve , . In the context of ULCPCI, this issue becomes especially relevant for various reasons. First, these patients often present with significant comorbidities (e.g., heart failure, valvular disease) and complex coronary anatomies, a situation in which it may be preferable to avoid the administration of adenosine. Second, as the use of contrast is limited, and for a precise mapping of the coronary tree several measures of pressures may be needed, repeated administrations of adenosine may be cumbersome. And finally, non-hyperaemic longitudinal vessel analysis allows for a precise determination of significant pressure drops along the vessel, identifying the target points to be treated with PCI. Currently available software such as SyncVision (Philips Medical, the Netherlands) can merge the information gathered from a pressure pullback using instantaneous wave-free ratio (iFR) and the fluoroscopic image and then display the drops of pressure along the vessel silhouette . Whilst this fusion image tool was first created to show iFR drops on top of a coronary angiogram, a ‘dry’ fluoroscopic image can be used, and the wire of the target vessel will define the path being followed by the pressure gradient (Figure 4). After PCI, physiology can also help determine the efficacy of revascularisation, and if a suboptimal result is identified, it will also aid in establishing if further interventions are needed (post-dilatation in underexpanded areas, additional stents in uncovered lesions, etc).
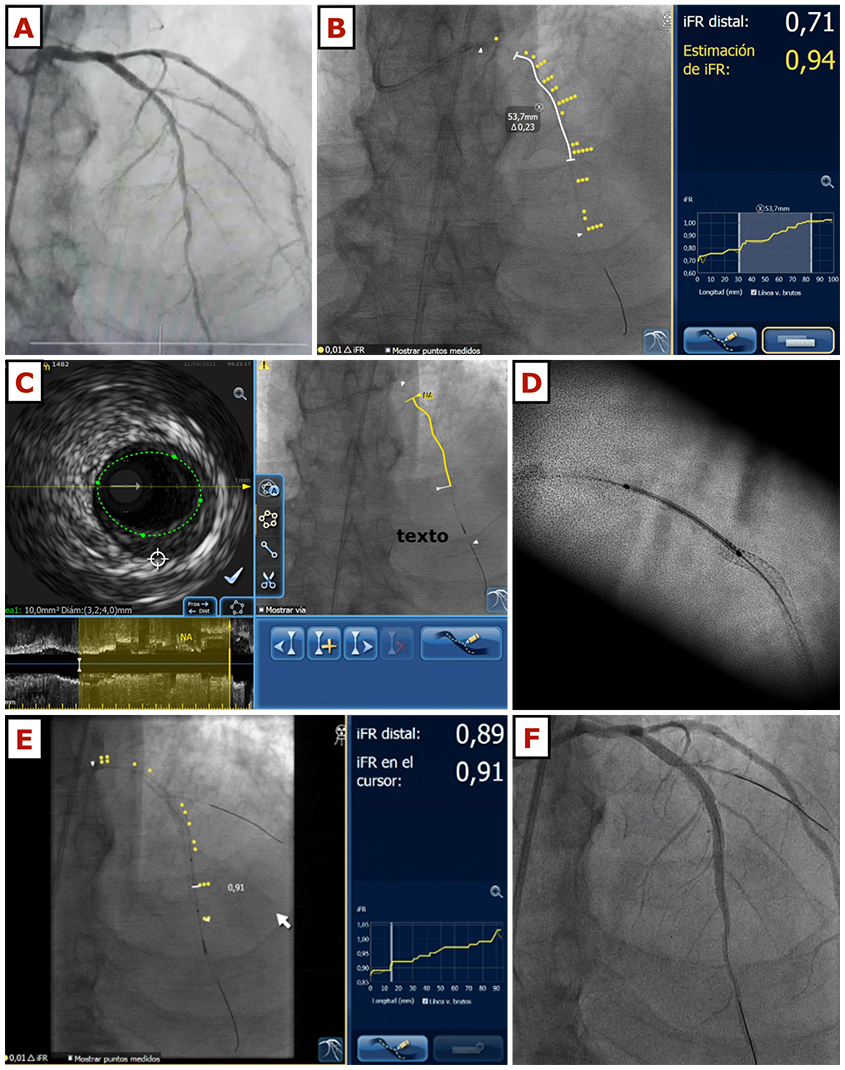
This figure illustrates the case of a 72-year-old gentleman with cardiovascular risk factors including hypertension and insulin-dependent diabetes mellitus, with stage 4 chronic kidney disease and an eGFR of 22 ml/min/m2. He was admitted with congestive heart failure and the echocardiogram showed left ventricular systolic dysfunction with an ejection fraction of 36%, anterior hypokinesis and moderate mitral regurgitation. A coronary angiogram using 18 ml of contrast was performed, showing a long, severe lesion in the left anterior descending artery (panel A). The patient was scheduled for ULCPCI and after catheter engagement and wire navigation using the previous angiogram as a reference, iFR measurements confirmed the stenosis severity. iFR pullback was co-registered with the fluoroscopic image using the wire as the marker of the vessel position (panel B). Then, intravascular ultrasound pullback was also done and co-registered with fluoroscopy (panel C). After predilatation with a 2.75 x 10 mm cutting balloon, the first drug-eluting stent was placed distally (2.75 x 20 mm). Then, a second drug-eluting stent was implanted. For a precise visualisation of the correct overlap area, stent-enhancing techniques were used, and no contrast was needed before the second stent (3.5 x 28 mm) was implanted (panel D). Post-dilation was done using a 3.5 non-compliant balloon and then, a pressure wire pullback was repeated (panel E) showing an excellent physiological result (iFR 0.91). A final injection was done in order to exclude any significant complications (panel F), and the procedure was finished, having used 8 ml of contrast in total.
eGFR: estimated glomerular filtration rate; iFR: instantaneous wave-free ratio; ULCPCI: ultra-low contrast percutaneous coronary intervention
In similar fashion to a pullback, using an intracoronary wire and pressure tracing, functional angiography tools such as Quantitative Flow Ratio (QFR®; Medis Medical Imaging, the Netherlands) can help to identify functionally relevant lesions along a vessel and the potential targets for revascularisation . Although the use of QFR and other angiography-based indices require contrast injections, they can be calculated using previous angiograms in an offline manner, thus limiting the need for subsequent interventions to ascertain the haemodynamic significance of coronary stenoses. The QFR software also allows for an estimation of PCI results via the quantification of residual QFR after a simulated removal of the obstruction . This can be compared to post-PCI iFR pullbacks to determine the success of revascularisation. An example of a pre-PCI QFR evaluation, the planning of PCI and a comparison with the final pressure pullback is shown in Figure 5.
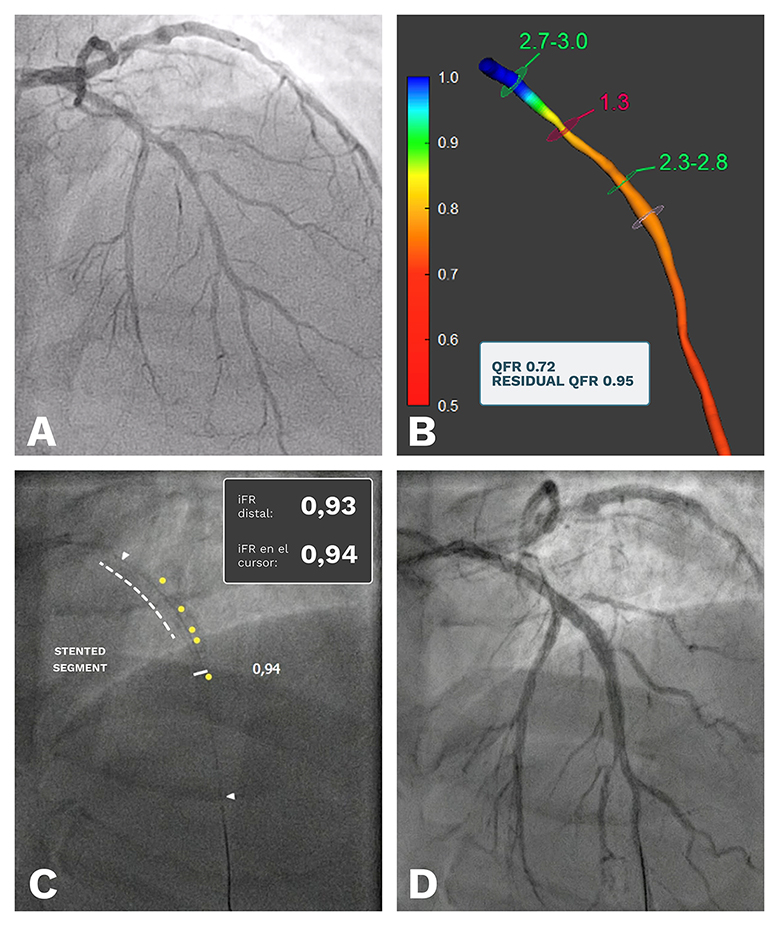
A case example of the use of Quantitative Flow Ratio (QFR) in the setting of ULCPCI. Using a previous angiogram (panel A), an offline QFR calculation was performed, showing a positive result (QFR 0.72) with the major estimated pressure drop located in the proximal segment of the left anterior descending artery (LAD). The simulation of a stent in that portion of the vessel yielded a significant improvement in vessel physiology (residual QFR 0.95, panel B), so PCI for this segment was scheduled. Using ULCPCI techniques, a stent was placed in the proximal portion of the LAD and after implantation the pressure pullback and co-registration showed an iFR of 0.94, very similar to the predicted QFR result (panel C). The final angiogram showed a good result without complications, and the procedure was ended having used 6 ml of contrast media. iFR: instantaneous wave-free ratio; ULCPCI: ultra-low contrast percutaneous coronary intervention
Along with coronary physiology, intracoronary imaging plays a role in ULCPCI. Because it does not require contrast to remove blood from the lumen, intravascular ultrasound (IVUS) may be the preferred choice for low-contrast dose interventions. The images gathered from IVUS pullbacks can precisely depict the coronary artery, giving a complete understanding of the lesion’s anatomy and characteristics. In a similar fashion to physiology, the automated IVUS pullback can also be co-registered- with the fluoroscopic image to select optimal landing zones and stent length (Figure 4). Repeating intravascular imaging after PCI offers information regarding the quality of the intervention in terms of stent expansion, lesion coverage, and edge complications. Evidence of the superiority of imaging-guided revascularisation compared to angiographic guidance is sound and reflected in current guidelines . The extensive use of intravascular imaging has also been shown to reduce the dependence on contrast administration, as highlighted in the MOZART (Minimizing cOntrast utiliZAtion with IVUS Guidance in coRonary angioplasTy) trial . In this study, patients were randomised to either angiography-guided or IVUS-guided PCI, with operators encouraged to use contrast-reduction techniques in both study groups. Patients randomised to the IVUS group received a significantly lower volume of contrast (20.0 ml vs 64.5 ml in the non-IVUS-guided group; p<0.001). No difference in the incidence of CIN was found, although this study was not powered for clinical outcomes. The ongoing and larger Mozart-II trial (ClinicalTrials.gov: NCT02743156) is powered to detect differences in CIN between the IVUS-guided and non-IVUS-guided study arms, and the results are expected to confirm the advantages of imaging-guided PCI.
Compared to IVUS, optical coherence tomography (OCT) has higher image definition and spatial resolution, but in general, contrast is used for image acquisition. However, experiences with OCT performed with agents other than contrast media have been reported, rendering images with comparable quality , . Future research in this field may pave the way for the adoption of OCT in ULCPCI practices, but so far, the limited evidence impairs its applications in clinical practice.
Following each angiogram, the excess contrast remaining in the guiding catheter may be removed by manual aspiration. This manoeuvre can, to a certain extent, diminish the amount of unnecessary contrast administered (e.g., during catheter exchange, flushing or drug administration).
Following this principle, contrast media can also be removed by aspiration of the coronary sinus right after intracoronary contrast injections. In a small non-randomised study of diabetic patients with CKD comparing coronary sinus aspiration versus standard care, this technique led to a 10% reduction in the amount of contrast media used. The incidence of CIN was lower in the coronary sinus aspiration group (5.5% vs 36.0%), although the procedural time was significantly higher in the intervention arm .
Other devices intended to minimise the use of unnecessary contrast have been tested but with limited results. The DyeVert™ PLUS (Osprey Medical, Minnetonka, MN, USA) is a system that is connected between the syringe and the manifold and diverges the excess of contrast into a dedicated container . The use of this device can reduce the amount of contrast administered but, as shown in the AVERT trial, it did not reduce the incidence of CIN in high-risk patients undergoing PCI .
The concept of ULCPCI, although initially conceived for patients with severe CKD, has now expanded to a wide range of scenarios, mainly in chronic coronary syndromes. However, as the adoption of this philosophy increases, so will the settings in which it can be implemented. The development of techniques and tools that minimise the use of contrast will continue to fuel the evolution towards minimal-dose or even zero-contrast interventions and will also involve other scenarios such as PCI in acute coronary syndromes or structural interventions like transcatheter aortic valve replacement (TAVR).
Ultra-low contrast percutaneous coronary interventions belong to the armamentarium of techniques and technologies that enable the performance of high-quality complex interventions possible, with the administration of minimal doses of contrast media. Its implementation requires a certain degree of operator experience and a broad use of technology, especially intravascular physiology, and imaging, which are a prerequisite for the assessment of coronary artery disease and the results of PCI (Figure 6).
_v3.jpg)
Key steps in the planning of ultra-low contrast percutaneous coronary interventions.
ACT: activated clotting time; CCTA: cardiac computed tomographic angiography; DAPT: dual antiplatelet therapy; eGFR: estimated glomerular filtration rate; iFR: instantaneous wave-free ratio. IVUS: intravascular ultrasound: PCI : percutaneous coronary intervention; QFR: quantitative flow ratio
With the adoption of ULCPCI practices, patients at risk of CIN can undergo indicated percutaneous coronary procedures and can be treated with the same high-quality standards as the vast majority of patients undergoing PCI.
Major changes in a discipline often are linked to revisiting its fundamental principles. This may well be the case of ultra-low contrast PCI: over more than 40 years of history PCI has remained indissolubly linked to coronary angiography, the only imaging method for coronary vessels available at the time of its inception. Considering an alternative approach to angiographic PCI guidance challenges what we learned during our training as operators and requires a change in mindset.
I was introduced to ultra-low contrast approaches nearly 20 years ago when working with Japanese colleagues performing CTO PCI. It is not a coincidence that such an approach was developed given the high utilisation in Japan. As they progressed in treating complex CTOs, it became clear to our Asian colleagues that conventional angiographic guidance was not feasible, either due to the risk of hydraulic dissection caused by contrast injections (for example, in CART procedures) or by the procedural complexity itself (liberal use of contrast is not possible in a 4-hour long intervention).
Like other interventionalists, I soon realised that, contrary to general belief, restricting contrast administration during PCI is important not only to perform safe interventions in patients with chronic kidney disease, but also to successfully complete a complex intervention without facing contrast volume limits, thus allowing enough time to adequately treat calcific vessels, CTOs, complex bifurcations or multivessel disease. In the context of treating patients with challenging clinical and anatomical profiles, there are multiple scenarios in which, nowadays, the safety and the quality of PCI may be improved by having the skills to perform large segments of the PCI procedure without contrast guidance. Ultimately, ultra-low contrast PCI is a truly contemporary approach which combines best practices such as image-based and intracoronary physiology, invasive and non-invasive imaging, and the proper use of radiology tools like dynamic coronary roadmapping or stent enhancement, with the aim of improving procedural and long-term outcomes.
In this chapter we share with you what we have learned and what we have put into practice every day in our cath lab. We are indebted to the many operators who have shared their experience with us. We hope you may find it useful and, even if you do not perform ultra-low contrast procedures, we hope that it prompts you to consider incorporating these skills into your practice: it only needs a change in mindset.
Evangelos Giannitsis, Christian W. Hamm, Holger M. Nef, Hugo A. Katus
Updated on May 14, 2022
Pim A.L. Tonino, Nico H.J. Pijls, Danielle C.J. Keulards
Updated on May 14, 2022
Javier Escaned, Daniel Faria, Asad Shabbir, Lin Wang, Shengxian Tu, Nieves Gonzalo, Hernan Mejia-Renteria, Xu Bo
Updated on May 14, 2022