Evangelos Giannitsis, Christian W. Hamm, Holger M. Nef, Hugo A. Katus
Updated on May 14, 2022
While the use of intracoronary pressure guidewires is currently recommended in clinical practice guidelines to assess functional stenosis relevance and the status of the microcirculation, adoption of functional coronary assessment remains being suboptimal. New developments in wire-free functional coronary assessment derived from the angiogram, globally termed as functional coronary angiography (FCA), may contribute to a more widespread use of physiology to diagnose the cause of ischemia and to plan and guide coronary revascularisation. In this chapter, we will revisit the principles and technical aspects of FCA, with a specific focus on techniques using invasive coronary angiography (ICA). Clinical applicability of FCA in specific clinical scenarios is also discussed.
Coronary angiography remains the most frequently utilised tool to plan and guide myocardial revascularisation, despite a large body of evidence highlighting its limitations in assessing functional relevance of coronary stenoses when used in isolation, particularly in cases of intermediate angiographic severity . The development of fractional flow reserve (FFR), a pressure-derived physiologic index, and its use in guiding percutaneous intervention, has led to improved patient outcomes compared with angiography guidance alone, triggering a step change in clinical guidance recommendations.Despite this, the widespread adoption of FFR has remained suboptimal . Reasons for the low penetrance of physiologic interrogation in routine clinical practice are largely inertial through the operator’s confidence in angiographic data and/or mistrust in coronary physiology, or perceived technical limitations including the reliance upon an adenosine infusion, extending procedural time and cost .
Over the last 10 years we have witnessed the development 2 different strategies to address the problem of the low uptake of FFR. Firstly, the development of wire-based non-hyperaemic pressure ratios (NHPR) which, by not requiring the use of coronary vasodilators, contributes to ease of use in the catheterisation laboratory whilst being non-inferior to FFR in terms of patient outcomes. NHPR have facilitated the widespread adoption of longitudinal vessel interrogation, a feature that, as discussed later in this chapter, has facilitated a more comprehensive approach to percutaneous coronary intervention (PCI) planning. Secondly, new developments have been made in the field of in-silico simulation of coronary haemodynamics. These systems are based on vessel lumen reconstructions from ICA or computed tomography coronary angiography (CTCA), with the ultimate aim of providing FFR-like estimates of stenosis severity, whilst not requiring pharmacological agents or intracoronary instrumentation. This latter approach, specifically FCA based on invasive angiograms, constitutes the focus of this chapter.
The 3 key steps required to derive vessel haemodynamics from coronary angiograms in FCA are; 1) selecting a fluid equation solver (computational fluid dynamics or simplified fluid dynamics equations), 2) generating a three-dimensional (3D) model of the coronary artery, and 3) defining physical boundary conditions , . (Figure 1)
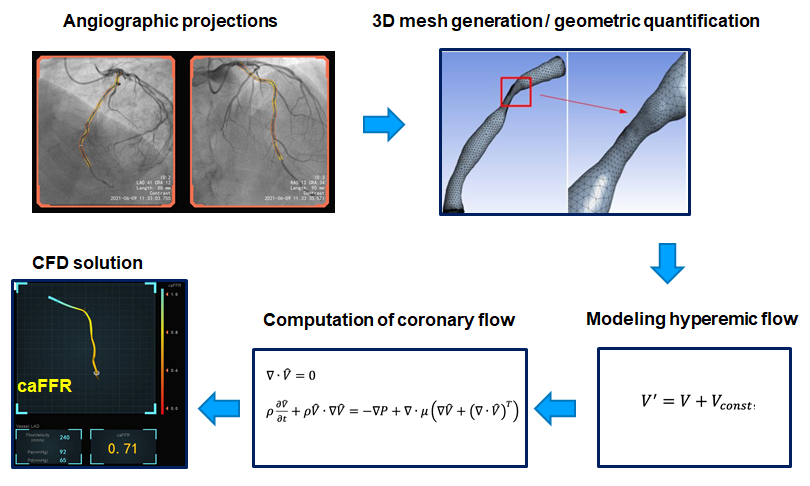
Key steps in functional angiographic analysis, exemplified with caFFR.
A fundamental aspect of FCA is the mathematical simulation of the effect of vessel anatomy on coronary flow. Navier-Stokes equations, the cornerstone of computational fluid dynamics (CFD), are frequently used to model the components of coronary blood flow velocity in FCA , . Despite the rheological properties of a non-Newtonian fluid such as blood perhaps interfering with the applicability of these equations, it is generally accepted that these calculations can be performed with a reasonable degree of accuracy based on assumptions of blood density and viscosity . However, CFD calculations can be time consuming and require considerable computing power. Simplified versions of CFD are therefore needed for online use , , .
Currently available FCA systems use 3D modelling to reconstruct the coronary lumen and to circumvent common sources of error associated with two-dimensional (2D) angiographic imaging, such as vessel foreshortening and lumen eccentricity. A mathematical 3D virtual mesh is generated from orthogonal ICA images, with the support of metadata embedded in DICOM files, which contain information on the position of the catheterisation laboratory table and image intensifier height, as shown in Figure 1 .
Flow in epicardial vessels is strongly modulated by shifting microvascular resistance, extravascular compression, and other factors specific to cardiac physiology. Simulation of flow should also include a mathematical description of haemodynamic factors at both the entrance and exit points of the analysed coronary segment (i.e. the inlet and outlet boundaries) that influence coronary flow . Information on the inlet boundary can be based on values of aortic pressure, which are measured from the guiding catheter or assumed based on averaged population data. Modelling the outlet boundary, which includes mathematical derivatives of the microcirculatory resistance and central venous pressure, is more complex. Whilst pre-set conditions are frequently assumed, as previously discussed, actual values of aortic pressure and flow velocity can be incorporated to the calculations to provide a more realistic and accurate estimation of boundary conditions , .
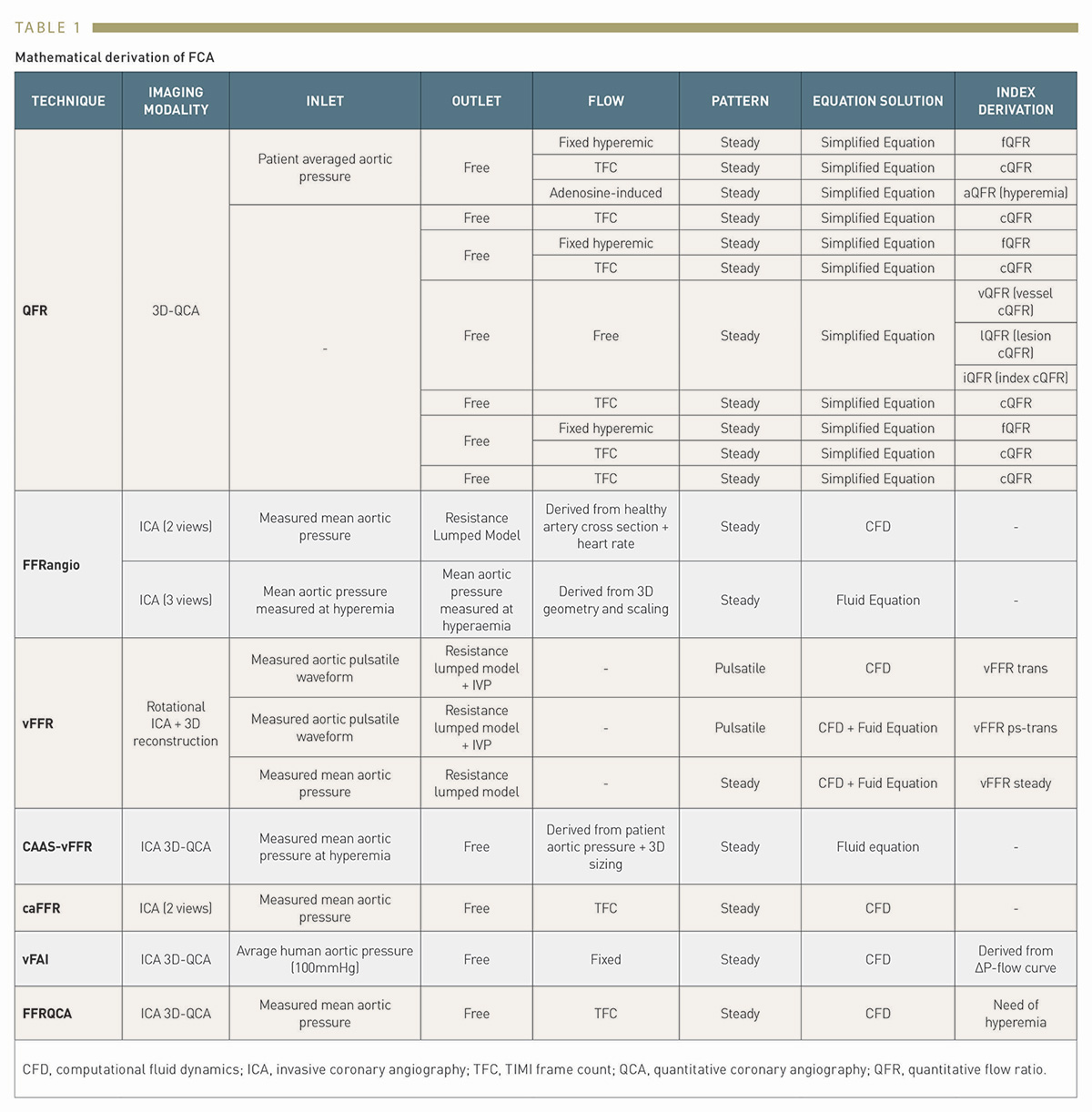
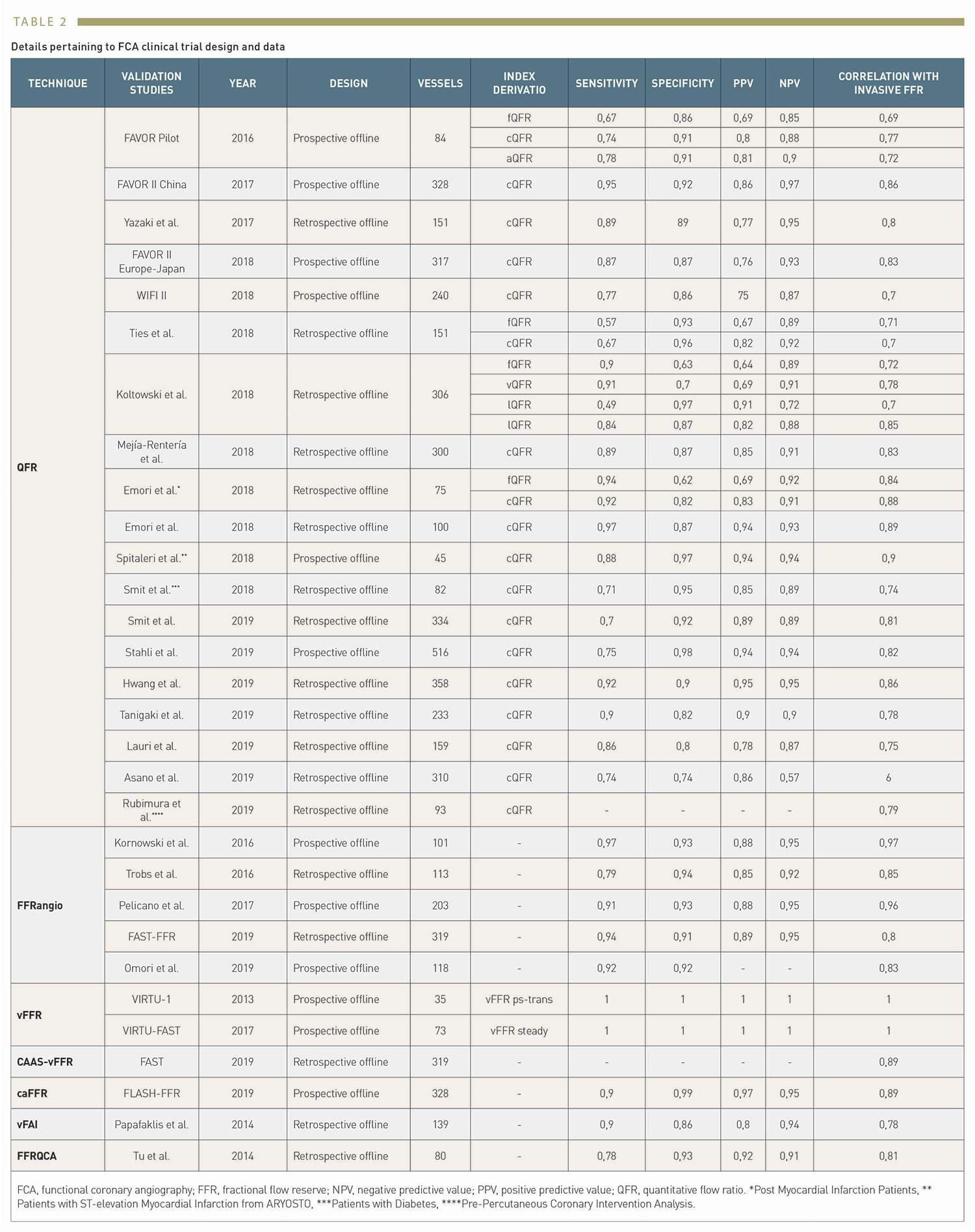
By not requiring invasive wire-based measurements, the induction of pharmacological hyperaemia or additional procedural costs, angiography-based techniques provide a solution to perform either online of offline functional evaluation of coronary stenosis in the catheterisation laboratory. Several different software approaches to FFR estimation derived from coronary angiography have been developed. Reported validation studies are shown in Table 1 and Table 2. In the following paragraphs we will discuss FCA systems that are currently approved for clinical use by regulatory agencies. (Figure 2)
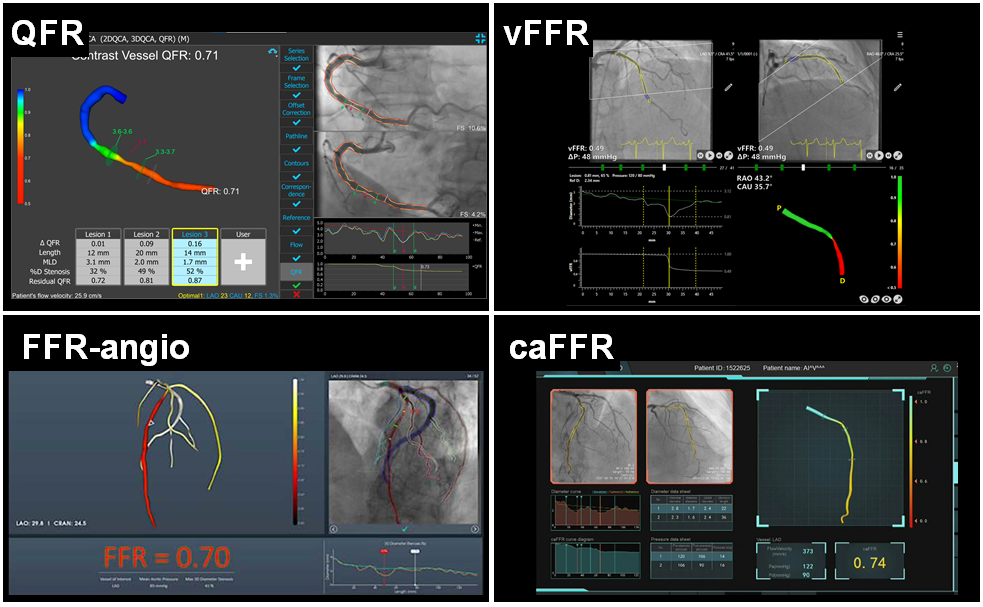
Commercially available functional coronary angiography systems: QFR, vFFR, FFR-angio and caFFR.
See text for details.
The most widely investigated FCA is Quantitative Flow Ratio (QAngio XA-3D, Medis Medical Imaging System, Leiden, the Netherlands and AngioPlus, Shanghai Pulse Medical Technology Inc., Shanghai, China). QFR is a computation method for calculating FFR, derived from 3D-quantitative coronary angiography (QCA), based on 2 orthogonal angiographic projections of the coronary vessel separated by at least 25º of angulation , . Once the vessel has been reconstructed into a 3D model, haemodynamic pressure drops are calculated at consecutive 6 mm segments according to derived friction and turbulence pressure coefficients, and subsequently integrated into the entire analysed segment. (Figure 3)
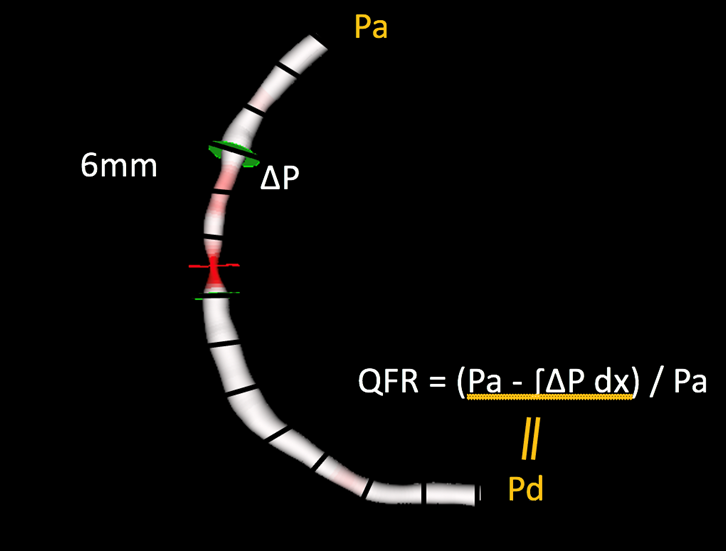
Fundamentals of the calculation of quantitative flow ratio (QFR).
Once a three-dimensional geometric mesh is derived from 2 orthogonal angiographic projections, partial pressure drops are calculated at consecutive 6 mm segments according to derived friction and turbulence pressure coefficients, and subsequently integrated into the entire analysed segment.
According to the type of flow value used for the calculation, QFR can be expressed in 3 different ways; 1) fixed-flow (fQFR) which utilises a fixed value of population-averaged hyperaemic flow of 0.35m/s, 2) contrast-flow (cQFR) that estimates flow velocity based on the TIMI frame count (TFC), or 3) adenosine-flow (aQFR) that measures flow from hyperaemic coronary angiograms obtained during adenosine administration . Most studies validating QFR have utilised the cQFR method, which offers the optimal balance between diagnostic yield and technical ease of use .
A new generation of QFR, termed as Murray bifurcation fractal law-based QFR (μQFR) was developed, allows computation of QFR from a single angiographic view and in both the main vessel and side branches (AngioPlus Core, Shanghai Pulse Medical Technology Inc., Shanghai, China). In the FAVOR II China study population, μQFR showed an excellent diagnostic concordance with wire-based FFR. The time of μQFR has been shortened to about one minute due to automation with artificial intelligence algorithms. (Figure 5)
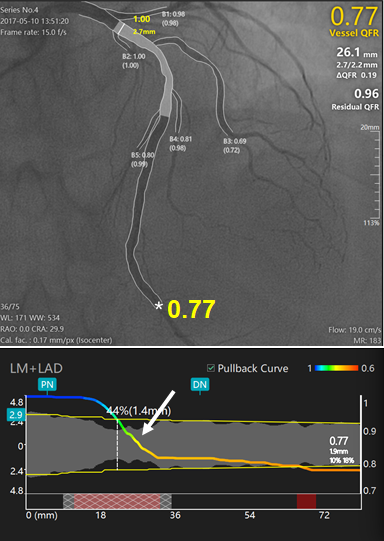
New generation Murray law-based QFR (µQFR) system powered with artificial intelligence algorithms.
Upper panel shows a diffuse intermediate lesion at the proximal to LAD. The lumen contours of LAD and its side branches were automatically delineated and superimposed on the angiographic images. The computed μQFR at the asterisk position was 0.77 while the wire based FFR was 0.78. Lower panel shows the co-registration between lumen size and μQFR pullback at every position along the LAD. The intermediate lesion correspond to a significant pressure drop in the μQFR pullbacks (white arrows).
Prior studies have demonstrated a good correlation between QFR and FFR values in angiographically intermediate lesions, with excellent diagnostic accuracy of QFR in the assessment of functional stenosis severity , , , , , , , , , , , , , , , , , , , . Two prospective, multicentre studies (FAVOR II China and FAVOR II Europe-Japan) have reported on the diagnostic accuracy of QFR at both the patient and vessel level , with better sensitivity and specificity compared to 2D-QCA in the assessment of the relevance of functional stenoses . In a patient-level meta-analysis of 16 high-quality studies comparing FFR and QFR, QFR demonstrated good positive and excellent negative predictive values in ascertaining the relevance of a coronary stenosis on the basis of an FFR cut-off of ≤0.80 .
QFR is the only functional angiography system for which the clinical value has been assessed in a randomised clinical trial. The FAVOR III China trial randomised 3825 patients with acute and chronic coronary syndromes, with at least one 50-90% coronary stenosis to a QFR-guided strategy PCI if QFR≤0.80) or an angiographic-based strategy. At 1-year of follow up, patients randomised to the QFR-guided strategy experienced better outcomes driven by fewer myocardial infarctions (MI) and ischemia-driven revascularisations (HR 0.65, 95% CI [0.51 to 0.83], P=0.0004) . The ongoing FAVOR III Europe Japan trial (NCT03729739) will report clinical outcomes associated with decision making on coronary revascularisation based on either QFR or FFR. A visual or a QFR analysis is shown in Figure 4.
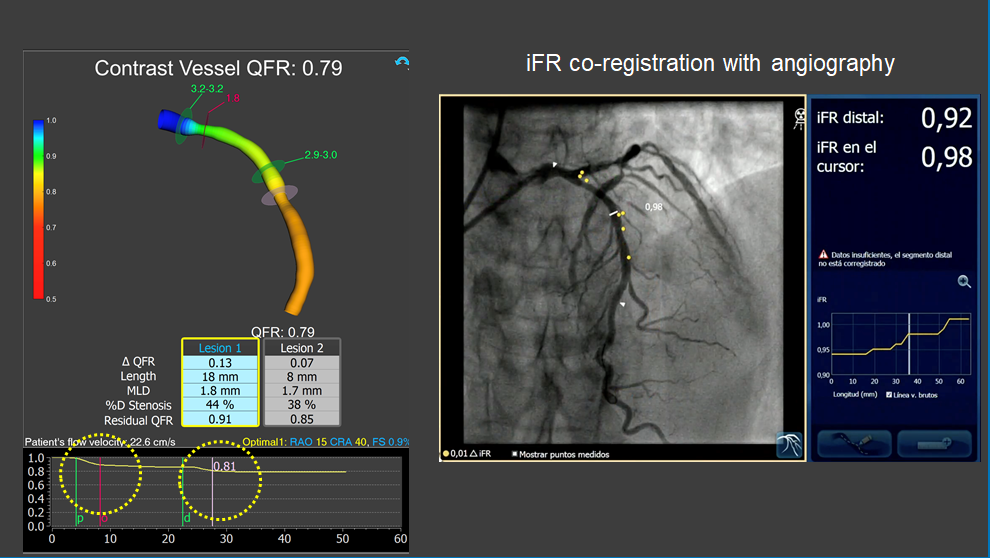
Assessment of intermediate LAD stenosis using QFR.
The analysis revealed two separate stenoses in proximal and mid LAD segments with mild-moderate flow-limiting effect (QFR = 0.79). iFR coregistration confirmed non-significant flow-limiting effect of coronary stenosis. Note the similarity between longitudinal vessel analysis obtained with QFR and intracororonary iFR pullback.
FFRangio (CathWorks Ldt., Kfar-Saba, Israel) involves the 3D digital reconstruction of the entire coronary arterial tree using at least 3 single-plane angiographic projections, and the estimation of flow by solving simplified fluid equations , . Microcirculatory resistance is estimated based on scaling formulas. The expected hyperaemic flow is estimated based on the calculated microcirculatory resistance and the total volume and length of the reconstructed coronary arterial system. FFRangio is subsequently obtained by dividing the expected hyperaemic flow rate in the stenotic artery by the hyperaemic flow rate calculated for a healthy vessel. The distinct advantage of FFRangio, compared with other invasive angiography systems, is that it is possible to make a simultaneous physiological assessment of the entire vascular tree, including side branches, in a single analysis.
Following small prospective offline studies validating and reporting good diagnostic accuracy for FFRangio , , the FAST-FFR trial, a large prospective multicentre study enrolling 301 patients, reported an excellent per-vessel sensitivity and specificity using invasive FFR as a reference. Interestingly, the diagnostic accuracy of FFRangio remained high even when only considering stenoses with ‘grey-zone’ FFR values of 0.75-0.85 .
Vessel fractional flow reserve (CAAS-vFFR, Pie Medical Imaging, Maastricht, the Netherlands) is based on 3D-QCA and simplified fluid equations. In CAAS-vFFR, the inlet uses an estimation of aortic root pressure at hyperaemia, and flow velocity is derived by applying the measured pressure to the reconstructed 3D coronary geometry. Based on the good linear correlation with wire-derived FFR in the FAST trial , the CAAS-vFFR system was the first invasive angiography-based physiology system to obtain Food and Drug Administration (FDA) market clearance. The recently reported FAST II trial results demonstrated that CAAS-vFFR has a high diagnostic accuracy to detect FFR≤0.80. The FAST III trial will address its prognostic impact compared with FFR and iFR (NCT04931771).
Computation pressure-flow dynamics derived FFR (caFFR, Raimed Ltd., Suzhou, China) uses a 3D angiographic reconstruction based on 2 orthogonal views, which are subsequently merged with CFD. Patient-specific aortic pressure, measured from the coronary catheter connected to a dedicated pressure sensor, is applied at the inlet boundary and resting flow velocities determined by the TFC method are integrated into the calculations to solve the Navier-Stokes equations . In the recently published FLASH FFR study, caFFR demonstrated adequate diagnostic accuracy to identify functionally significant lesions, with wire-based FFR as a reference .
Estimated procedural PCI risk based on the SYNTAX score, which is derived from an angiographic assessment, is markedly modified when only functionally relevant coronary stenoses are entered in the calculation of the index, a concept named functional SYNTAX score, which was first proposed for wire-based FFR. Asano et al. derived a wire-free functional SYNTAX score based on QFR (fSSQFR) by retrospectively screening and analysing all lesions interrogated by iFR/FFR in the SYNTAX II trial. According to the 2-year patient-orientated composite endpoint (all-cause death, MI, or any revascularization), fSSQFR reclassified 26.1% of patients into the low-risk group (net reclassification improvement 0.32, P<0.001) and its accuracy to predict this composite endpoint was higher than that of the classic anatomical SYNTAX score (0.68 versus 0.56, P=0.002).
Contrary to single physiological measurements performed in the distal segment of the interrogated vessel, longitudinal physiological assessment of the vessel allows characterisation of the flow-limiting effect of each stenosis located along the coronary artery, providing an additional dimension to the physiological analysis. FCA tools provide a unique opportunity to perform this type of analysis, and for co-registering physiological an anatomical information. (Figure 6) Accurate length measurements can be obtained as part of the QCA features of FCA systems.
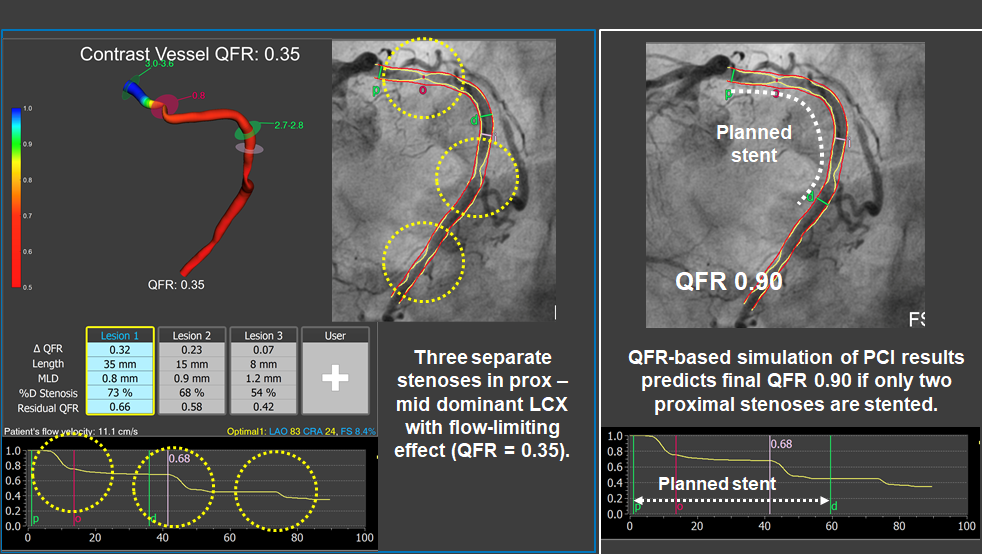
Simulation of functional results of PCI using QFR.
A dominant left circumflex artery presented three separate stenoses with a combined flow-limiting effect (QFR = 0.35). By calculating the residual QFR value, it was possible to predict that the more distal stenoses would have no significant functional impact if the two proximal ones were treated (estimated QFR 0.90). After following that revascularisation plan, leaning un-stented the more distal stenosis, final iFR in the LCX vessel was 0.93.
In planning PCI, focal or diffuse patterns of flow-limiting disease can be established through visual or mathematical analyses of longitudinal physiological assessment. Longitudinal vessel analysis allows identification of focal and diffuse patterns of flow-limiting disease, either by visual inspection of the pullback curve or by using the pressure pullback gradient index (PPGindex). The pressure pullback gradient index (PPG), which has been proposed to analyse FFR pullback curves and aid in the differentiation of disease pattern subtypes, has been applied to QFR.
Longitudinal physiological vessel interrogation is also useful after PCI, as it may identify residual focal pressure gradients inside or outside the stent. When such pressure losses are focal, additional stent post-dilatation or PCI may be considered to optimise the functional results.
Simulation of functional PCI results based on longitudinal vessel analysis is an important aspect of PCI planning, and readily available with FCA systems. Post-PCI physiology correlates with target vessel failure, and therefore the prediction of success of PCI prior to embarking on deployment of a stent is appealing. Although PCI simulation technologies are established for wire-based pressure indices, in-silico simulation with FCA has also been tested in clinical studies. QFR has demonstrated a good correlation between predicted residual QFR (pre-PCI) and post-PCI QFR and FFR. The clinical utility of this virtual PCI tool with QFR will be assessed in the prospective AQVA study (NCT046641040). A similar tool has also been developed for angiography derived vFFR to predict the physiological effects of PCI, using CFD and proprietary software (VIRTUhear[TM] workflow system, United Kingdom) with a high degree of accuracy, when compared to invasive FFR as a comparator.
While the other sections in this chapter have focused on the use of FCA in the assessment of obstructive disease in the epicardial coronary vessels, contemporary research suggests that its diagnostic value might also extend to the assessment of the coronary microcirculation. Estimations of intracoronary pressure and vessel flow can be derived from the aortic pressure, QFR and TFC. Using this approach, De Maria et al. developed and validated an angiography derived index of microcirculatory resistance (IMRangio) that requires the use of angiographic projections with hyperaemia. Tebaldi et al. have validated another angiography-based index of microcirculatory resistance (A-IMR), utilising IMR as a comparator, in patients with chronic coronary syndrome with an intermediate LAD lesion, demonstrating a good correlation between both indices. Further research by Mejía-Rentería et al. identified that non-hyperaemic angiograms can be used to calculate angio-IMR, provided a correction factor is introduced in the calculation of intracoronary pressure. (Figure 7)
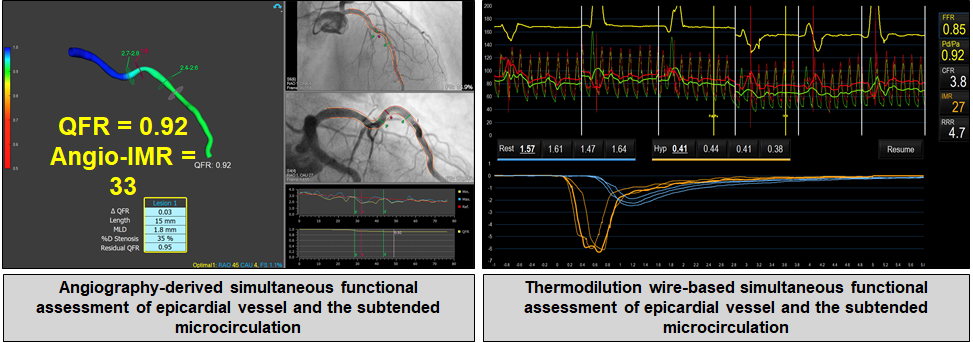
Assessment of microvascular resistance with functional coronary angiography.
Left panel: Three-dimensional model of a coronary artery (LAD, left anterior descending) with computational simulation of transtenotic pressure ratio (i.e., QFR, quantitative flow ratio) for functional assessment of coronary stenosis (mid segment) and the microvascular resistance (angio-IMR). Right panel: Wire-based functional assessment of the same coronary vessel with simultaneous measurements of transtenotic pressure ratio (i.e., FFR, fractional flow reserve) and thermodilution-derived coronary flow. In this patient, both techniques revealed non-obstructive coronary stenosis at mid LAD (QFR and FFR > 0.80), but coronary microvascular dysfunction as determined with microcirculatory resistance (both angio-IMR and IMR ≥ 25).
Most of the studies commented on thus far have been performed in patients with stable coronary disease with intermediate stenoses located in a major coronary vessel. However, research has also been conducted to explore the value of FCA in in other clinical and anatomical scenarios. These are discussed below in the following paragraphs.
In patients with acute coronary syndrome (ACS), 2 distinct areas of research have been conducted: 1) assessment of non-culprit stenoses and 2) assessment of microcirculatory injury downstream the infarct related vessel.
Approximately half of the patients presenting with ST-segment elevation myocardial infarction (STEMI) also have bystander multivessel coronary artery disease and non-culprit lesions (NCL). Repetitive invasive assessments of these vessels is associated with increased risk, cost, and time, especially in non-functionally significant NCL. The ability to estimate the functional relevance of NCL offline after primary percutaneous coronary intervention (PPCI) could change the management of these patients, enabling decision making regarding further PCI without a need for additional invasive procedures, thereby reducing the risk of procedure related adverse events to the patient. (Figure 8).
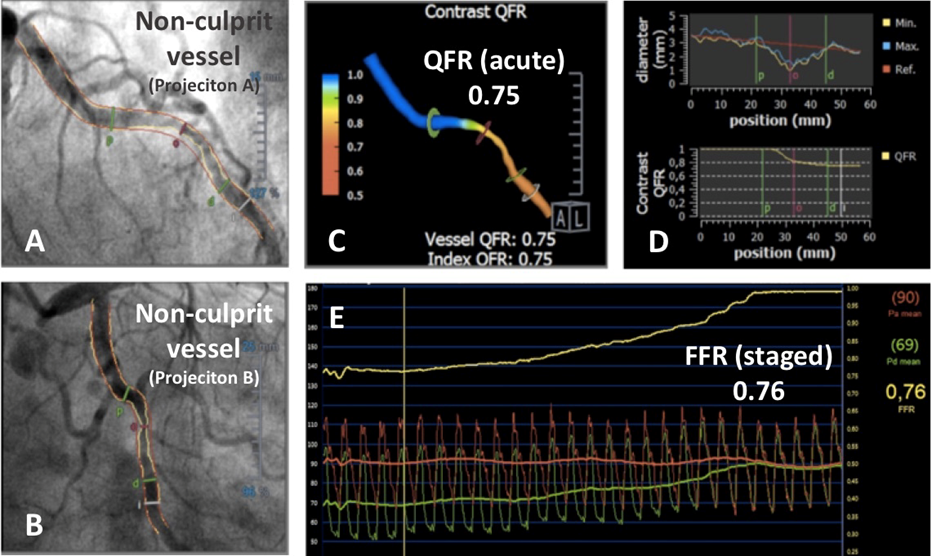
Use of QFR to analyse a non-culprit stenosis in a patient presenting with ST elevation myocardial infarction (STEMI) and multivessel disease.
A non-culprit lesion in circumflex artery assessed by QFR using the angiogram obtained over the acute phase of STEMI (A-B). A three-dimensional reconstruction of the vessel with colour mapping of the pressure drop (C). Longitudinal vessel analysis with QFR revealed the presence of a flow-limiting stenosis (D). An FFR pullback performed five days later in the same vessel over a staged angiogram confirmed QFR findings made at the acute STEMI phase (E).
Overall, available evidence is concordant and supports the value of QFR for this indication. Lauri et al. published a retrospective, observational, multicentre study demonstrating that QFR has excellent diagnostic accuracy for the assessment of the functional relevance of NCL during PPCI. Sejr-Hanse et al. performed a post-hoc analysis of the iSTEMI study demonstrating that acute-phase QFR has a very good diagnostic performance, with staged FFR as reference. Spitaleri et al., in a proof-of-concept study performed invasive FFR and QFR in patients with STEMI with NCL and found an excellent correlation and agreement between QFR and FFR values in both acute and staged procedures. Patients with a QFR value of ≤0.80 were at high risk of adverse events (HR 2.3, 95% CI 1.2-4.5, P=0.01).
Choi et al. demonstrated that QFR consistently shows high diagnostic performance to predict the functional significance of epicardial coronary stenosis regardless of vessel location and lesion length, in various clinical presentations such as in ACS NCL (n=153), previous MI (n=30), or diabetes mellitus (n=190). Erbay et al. studied the prognostic impact of QFR analysis in post-interventional culprit and non-culprit vessels in 792 patients with ACS. The results showed that abnormal QFR is an independent predictor of 2-year MACE in NCL (OR 3.78 [95% CI, 2.21 to 6.45], P<0.001) and post-PCI culprit vessels (OR 3.60 [95% CI, 2.09 to 6.20], P<0.001).
Regarding the use of FCA to assess microcirculatory injury downstream of infarct related vessels, Choi et al. confirmed the prognostic value of an elevated angio-IMR calculated from caFFR and pressure simulations in 2 cohorts of patients with STEMI that were followed-up for 10 years. These data have demonstrated that patients with an angio-IMR>40 experienced significantly increased risk of cardiac death and hospitalisation for heart failure.
The performance of QFR in in-stent restenosis (ISR) has been evaluated retrospectively in a multicentre study by Liontou et al., and shown agreement between FFR and QFR, comparable to values reported with de-novo lesions. Additionally, the reported performance of QFR to establish ISR relevance, with wire based FFR as reference, was high (AUC 0.90, 95% CI 0.83-0.97).
The prognostic impact of coronary revascularisation in patients with aortic stenosis undergoing surgical (SAVR)or transcatheter (TAVI) valve replacement remains unclear. This puts more weight on ensuring that coronary stenoses are functionally relevant before embarking in PCI or surgical treatment. Most recent guidelines recommend considering PCI in patients with a primary indication to undergo TAVI and coronary artery diameter stenosis >70% in proximal segments (IIa recommendation, C level of evidence). Stenosis assessment with wire-based indices like fractional flow reserve (FFR), instantaneous wave-free ratio (iFR) or resting full-cycle ratio (RFR) is challenging in these patients, since intracoronary measurements made as part of diagnostic work-up might shift once boundary conditions like left ventricular pressure overload are modified by TAVI or SAVR.
In this context, FCA might have the advantage that, at a difference with pressure-based indices, estimates of stenosis severity should remain unaffected by such changes in physiological boundary conditions. A number of studies have assessed the diagnostic performance of quantitative flow ratio (QFR) in patients with AS, showing an overall good correlation between baseline QFR stenosis values and FFR measured either before or after TAVI. One of the studies revealed that the correlation between QFR and FFR values before TAVI in patients with very severe aortic stenosis decreases, a fact that, potentially, might reflect more the effect of microcirculatory compression on FFR assessment than on the ability of QFR to predict stenosis relevance. Future studies will clarify the ability of FCA to predict post-TAVI functional stenosis relevance.
By assessing the location of flow-limiting stenosis, functional coronary angiography may contribute to better PCI planning and to avoidance of residual disease after PCI caused by anatomical mismatch. Longitudinal QFR curves obtained in vessels satisfactorily treated with PCI in the HAWKEYE study revealed that a suboptimal functional result had occurred in approximately 16% of cases. The cause of the abnormal QFR values were a combination of focal in-stent (13%), focal outside-stent (32%) and diffuse (34%) disease. The remaining patients experienced combined residual disease patterns post-PCI.
These findings are in agreement with those from the DEFINE PCI study, which also detected a high prevalence of residual flow-limiting disease after routine PCI. The HAWKEYE investigators also observed that abnormal QFR values post-PCI infers a poor prognosis, with a 3-fold increase in vessel-oriented composite endpoint (cardiovascular death, MI or ischemia-driven target vessel revascularization) in vessels with offline post-PCI QFR≤0.89 (HR 2.91, 95% CI 1.63-5.19, p<0.001) and significant differences in all three composites. The prognostic utility of post-PCI FCA has also been supported by recently published data from the PANDA III study, which identified that a low post-PCI QFR value of ≤0.92 was associated with a higher risk of 2-year vessel-orientated composite outcomes (HR 5.5, 95% CI 3.03-10.0).
Most of the current versions of the angiography-derived techniques are operator dependent and require several manual steps (manual indication of landmarks, correction of vessel contours and start/ending of the vessel of interest), which might account for significant interobserver variation in clinical settings,. As accuracy depends directly on the quality of the acquired images and optimal projections, overlapping or highly tortuous vessels cannot be optimally evaluated. In systems where TFC is used to estimate coronary flow, low quality contrast injections might negatively influence the accuracy of the data. The lack of standardised contrast injections may account for differences in reported correlations with invasive FFR.
Misalignment between boundary and actual conditions may account for discrepancies between FCA and intracoronary physiology measurements. Microcirculatory dysfunction, as defined by IMR≥23, decreases the diagnostic performance of QFR to detect functionally significant lesions as determined by FFR. In the same direction, discordance between QFR and iFR values tend to occur when coronary flow reserve (CFR) is impaired, and disagreement between resting Pd/Pa and FFR functional classification is associated with reduced diagnostic accuracy of QFR. All this may explain discrepancies between FFR and QFR reported in patients with conditions that may affect the coronary microvasculature, like chronic renal disease and diabetes mellitus. Nevertheless, QFR is still superior to angiography alone in ascertaining the functional severity of a stenotic coronary vessel in patients with microvascular disease. Developments in the use of FCA to assess the coronary microcirculation discussed earlier in this chapter may contribute to increase the diagnostic yield of FCA when used to assess stenosis severity in the presence of microvascular disease.
The use of invasive angiography derived FCA has the potential of circumventing many of the limitations associated with conventional wire based hyperaemic and non-hyperaemic pressure indices. Trial-based evidence support that, compared with angiography-based decision making, FCA contributes to better functional coronary revascularisation and improves patient outcome In addition to setting the indication of PCI, FCA provides opportunities for adequate planning and guidance of revascularisation procedures Pre-PCI assessment with FCA has been demonstrated to aid in clinical decision making through longitudinal vessel analysis, with novel insights into simulation of PCI whilst also predicting prognosis through the evaluation of post-PCI residual flow-limiting disease. Finally, there is consistent evidence that FCA may provide information as to the status of the coronary microcirculation, thus facilitating its use to evaluate the status of this vascular compartment.
Evangelos Giannitsis, Christian W. Hamm, Holger M. Nef, Hugo A. Katus
Updated on May 14, 2022
Pim A.L. Tonino, Nico H.J. Pijls, Danielle C.J. Keulards
Updated on May 14, 2022
Alejandro Travieso, Breda Hennessey, Asad Shabbir, Adrian Jerónimo, Nieves Gonzalo, Javier Escaned
Updated on May 11, 2023