Horacio Medina de Chazal, Ali Zgheib, Angelo Quagliana, Michael Chetrit, Jean Buithieu, Giuseppe Martucci, Marco Spaziano, Ali Abualsaud, Ole de Baker, Laurence Campens, Pascal Theriault-Lauzier, Jere...
Updated on November 23, 2022
Tricuspid regurgitation (TR) increases with age, reaching about 4% of patients 75 years old, and is associated with increased risk of cardiovascular events and death at long-term follow-up.
Functional TR is the most frequent form of TR, due to the remodeling and dysfunction of right heart chambers resulting in tricuspid annulus dilatation and dysfunction with or without associated leaflet tethering. Often, it is observed in patients with right ventricle remodeling and pulmonary hypertension, usually secondary to left-heart valvular disease (with or without previous surgical correction, “ventricular TR”). It also develops following tricuspid annulus dilatation/dysfunction in the setting of atrial fibrillation without left-heart disease (“atrial TR”).
Echocardiography is the cornerstone of TR assessment, including regurgitation severity, valve anatomy and right ventricle function. Alongside this, cardiac computed tomography and right heart catheterization are used increasingly to adequately selected patients and plan the procedures.
Depending on the specific tricuspid apparatus, numerous and very different transcatheter therapies are available to address different targets, including leaflet approximation, annuloplasty, valve replacement, valve-in-valve implantation and heterotopic valve implantation. Such devices are still in an early phase of development, but initial evidences are favorable and promise to allow more patients affected by TR to receive a much needed treatment.
Tricuspid valve disease has recently being put under the spotlight and its historical knowledge is currently getting refreshed by new evidences.
While tricuspid stenosis is very rare in western countries, the prevalence of significant TR (moderate or more) in the general population is estimated 0.55% and it increases with age being 1% above 65 years and 4% above 75 years . Of note, historically only 2.6% of these patients received surgery, and only in the presence of advanced heart failure despite medical therapy.
TR is classified as organic (primary) and functional (secondary). A third category made up by patients presenting interference with a cardiac implantable electronic device (CIED) is emerging as deserving specific consideration: these cases present a multifactorial pathophysiology and share features of both primary and secondary TR. This group currently represents <5% of all TR cases but it is expected to increase in the future , .
Primary TR is observed in a minority of patients (5-10%), and is associated with organic lesions of the leaflets and chordae, including numerous aetiologies: congenital, infective endocarditis, neoplastic, rheumatic, endomyocardial fibrosis, traumatic or iatrogenic causes (right ventricular biopsy).
Functional TR is the most frequent form of tricuspid valve disease (80-90% of all TR patients) and its prevalence is increasing. Functional TR is caused by lack of leaflet coaptation in the absence of organic valve lesions and can be further sub-classified, Figure 1 , . Most often (70-75% of functional cases), it develops progressively following right ventricle remodeling in patients with left-sided heart valve disease or pulmonary artery hypertension or right ventricle disease (“ventricular TR”); the regurgitation mechanism typical of this phenotype is predominant leaflet tethering due to the remodeling of the right ventricle, which can be associated to annular dilatation; the right ventricle is severely remodeled and dysfunctioning. Functional TR may also be secondary to long standing atrial fibrillation causing right atrial and tricuspid annulus remodeling and dysfunction (“atrial TR”). In this setting the main regurgitation mechanism is annular dilatation without significant leaflet tethering; the right ventricle is usually only mildly dilated and not severely dysfunctioning.
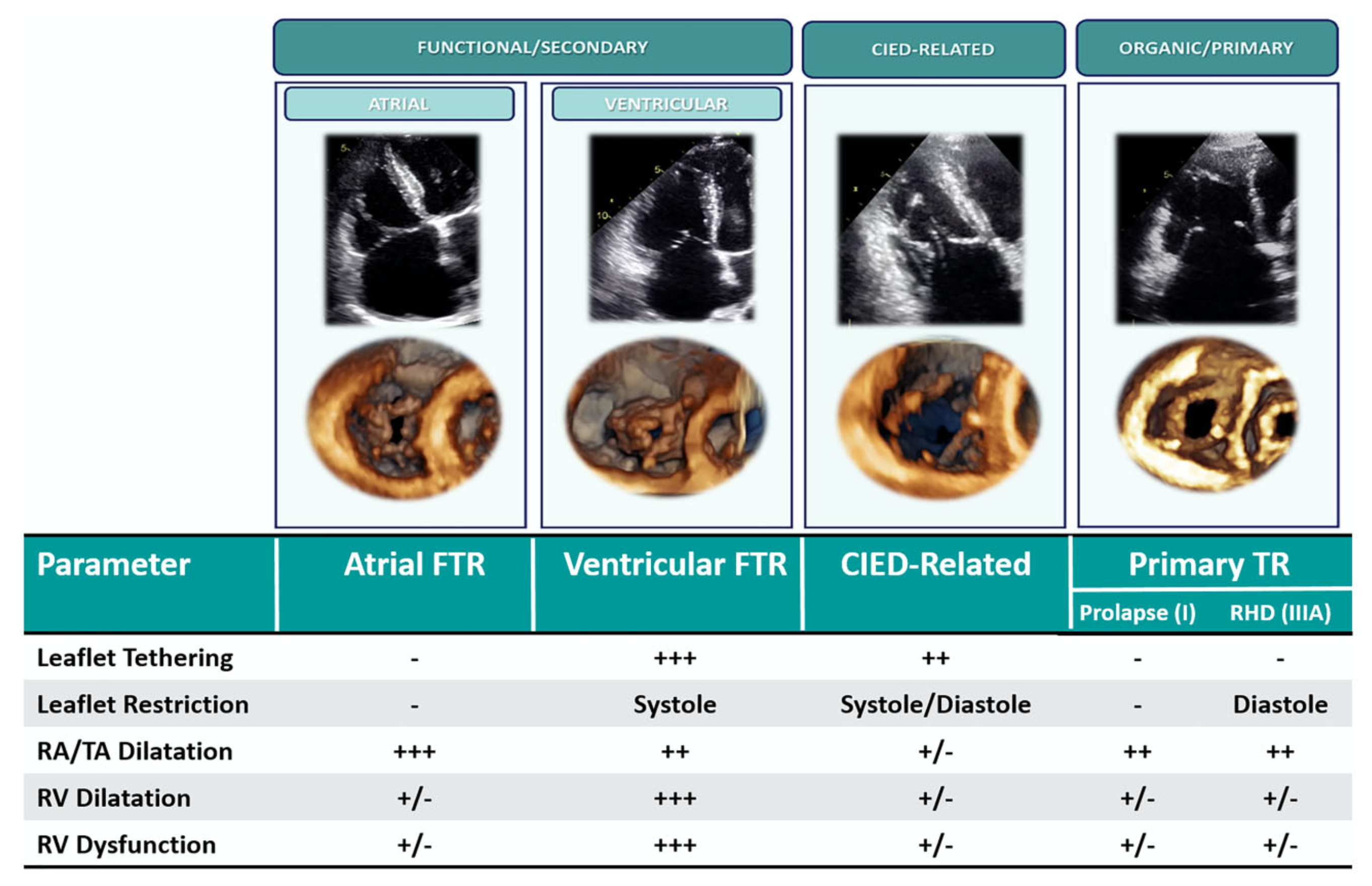
Classification of TR.
Primary TR: organic disease of valve leaflets is present (prolapse, rheumatic disease, endocarditis, other). The right atrium is dilated; the right ventricle can be dilated and/or failing depending on the stage of the disease. Cardiac Implantable Electronic Device Related: interference with a lead causes TR; features of primary and secondary TR can both be present. Secondary (Functional) TR, sub-classified in atrial and ventricular TR. Atrial TR is due to atrial fibrillation causing right atrium and tricuspid annulus dilatation/dysfunction; no severe leaflet tethering nor right ventricle dysfunction are usually present. Ventricular TR is due to left-side heart disease / pulmonary hypertension / right ventricle disease causing right ventricle dilatation and dysfunction; leaflet tethering is typically observed along with significant right ventricle remodeling. Reproduced with permission from Hahn et al. .
TR is particularly frequent in patients with mitral valve disease . TR tends to progress after mitral surgery if left untreated , and also in patients undergoing concomitant TR repair at the time of mitral surgery persistent/recurrent TR may still present to various degrees depending on the type of tricuspid repair performed , , .
TR per se is associated with reduced survival and increased hospitalization due to heart failure , , . Patients experience progressive reduction of functional capacity and eventually severe disability despite medical therapy.
Tricuspid regurgitation is a deceitful disease. In the absence of pulmonary hypertension, TR is well tolerated for very long time and that has led to neglect the tricuspid valve in the past. Diuretics and vasodilators are indeed effective in reducing symptoms in this setting but progression of right ventricle remodeling steadily and slowly continues. Unfortunately at later stages, when medications are no more effective in symptoms control, it is often already too late to intervene because at this time the right ventricle dysfunction is too advanced. This is indeed the timing at which patients had been referred to surgery in the past and this is the reason of the dreadful mortality reported in historical surgical series. , , . Older age, severe hepatic and/or renal impairment, ascites, anaemia and albumin before intervention are independent determinants of surgical hospital mortality.
Notably, recent evidences demonstrate that the modern overall acute surgical mortality of 6% can actually be brought down to 0% in case of early surgery while it can reach as high as 15% in case of late referral . The same is observed in the long-term, with 5-year survival ranging from 100% of patients operated in early stages down to 60% for patients treated in advanced stages .
Recently new scores to predict patients’ survival and the operative risk of mortality of tricuspid surgery have been proposed.
The fact that 2021 Guidelines recommendations for surgery are still currently largely based upon expert opinion in the absence of solid evidence-based trial data describes the delay we still have in the tricuspid field, Figure 2. In case of concomitant to left-side operation, surgery is indicated in case of severe TR or in case of mild-moderate TR with concomitant tricuspid annulus dilatation (≥40 mm or >21 mm/m2). Without concomitant left-side indication, surgery is recommended in case of severe TR with symptoms and right ventricle dilatation without severe ventricular dysfunction nor pulmonary hypertension . When appropriate, surgical valve repair is always preferable to valve replacement. Within repair, rings have provided more durable TR correction compared to suture annuloplasties .
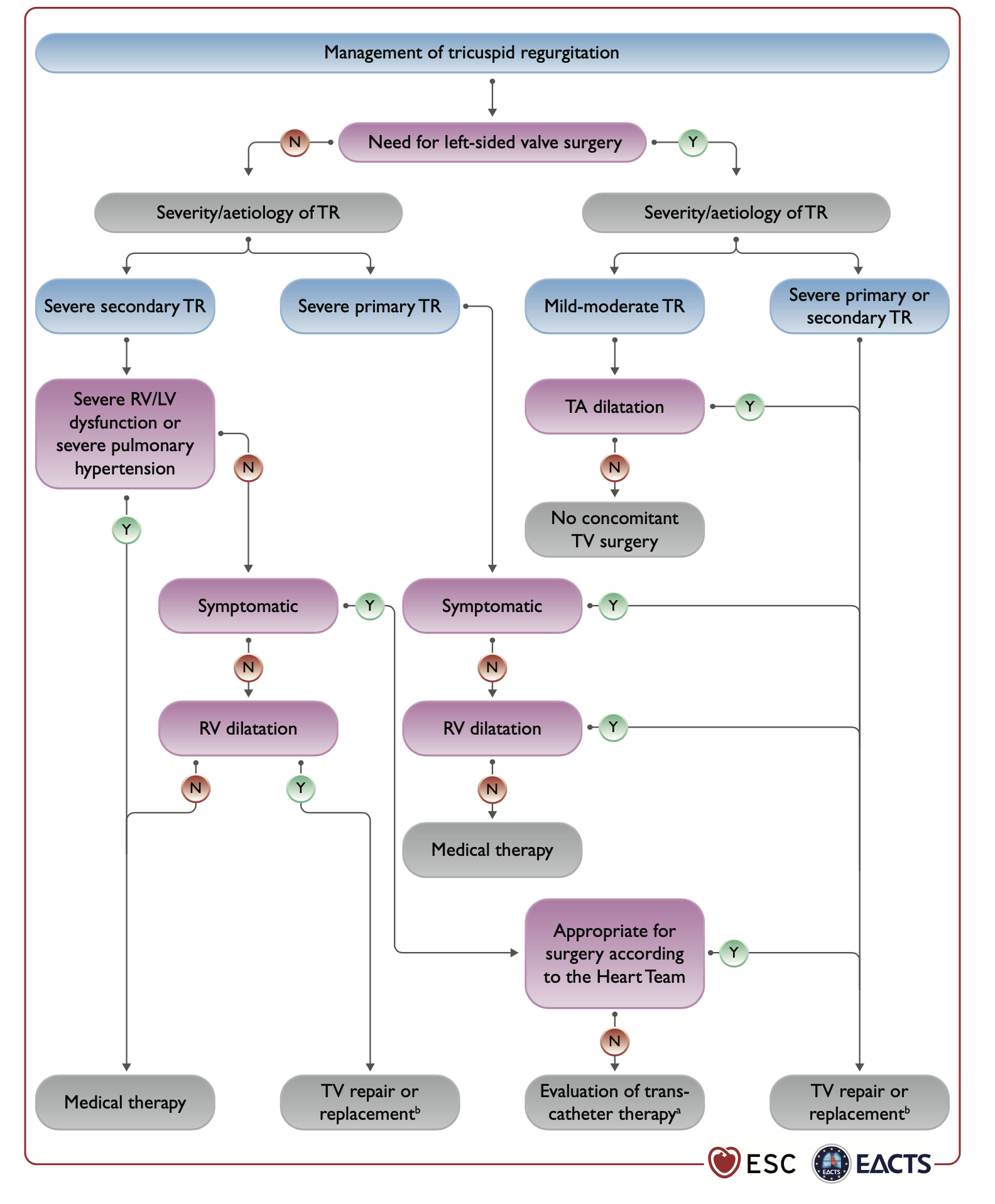
2021 ESC/EACTS Guidelines recommendations for TR management.
Reproduced with permission from Vahanian et al. .
As in previous other settings, percutaneous tricuspid procedures emerged as an attractive alternative for patients with symptomatic tricuspid disease at increased surgical risk. Such use is supported by the 2021 European Guidelines, provided that the procedure is conducted at a Heart Valve Centre with high level of expertise .
As previously mentioned, a big number of tricuspid patients present in a late stage, when the risk of surgery is high or they are not even referred at all due to perceived too high procedural risk. All these patients represent current potential candidates of percutaneous tricuspid therapies.
Similarly to surgery, an important group of patients is given by those affected by concomitant mitral disease and treated with transcatheter mitral therapies (especially edge-to-edge repair). Moderate-to-severe TR at baseline is frequent in these patients (47 out of 146 consecutive patients in the GRASP registry) and has a negative impact on the 30-day and 12-month outcome on the combined risk of death and rehospitalisation for heart failure . In this specific population, a simultaneous or staged percutaneous treatment of TR could be a safe option to improve outcomes. Available data suggest that improvement of TR after mitral TEER occur in approximately 50% of patients with moderate or more TR at baseline but larger data together with the understanding of predictors of TR improvement after the mitral procedure will be needed to clarify patients who could benefit from concomitant or second-step tricuspid correction.
In-depth knowledge of the complex surgical anatomy of the TV is a mandatory prerequisite to understand the challenges, which are encountered in developing percutaneous tricuspid therapy.
The tricuspid is the bigger and more anterior valve of the heart.
The normal TV apparatus is composed of three leaflets (septal, posterior and anterior), chordae tendinae, and two or three well developed papillary muscles. The posterior and anterior leaflets could be considered scallops of a single mural leaflet, similar to the posterior leaflet of the mitral valve. Variable distribution of the scallops is possible and more than three leaflets can be identified in about 50% of the patients, especially depending on the pattern of the subdivision of the mural leaflet. The number of leaflets observed in daily practice is so variable that a new detailed tricuspid anatomy classification which describes four different types of valve morphologies based upon leaflet sub-divisions has been proposed , Figure 3. This leaflet classification is particularly useful for transcatheter devices which target the edge of native leaflets.
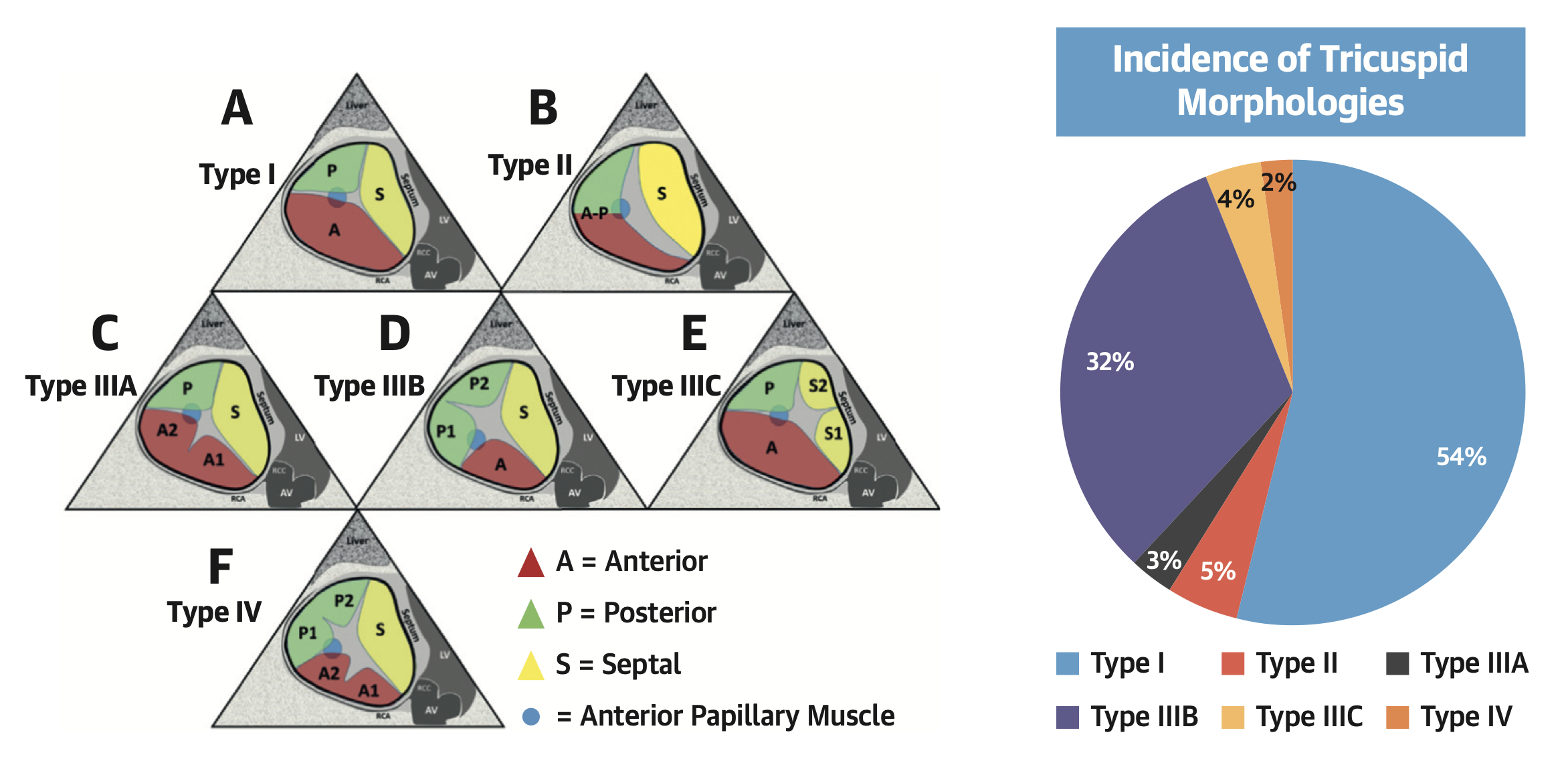
Anatomical classification of the tricuspid valve based on leaflet morphology.
Various clefts, indentations, divisions of tricuspid leaflets are usually observed in clinical practice. Reproduced with permission from Hahn et al. .
Papillary muscle anatomy is also variable, with a septal and an anterior papillary muscle. The septal leaflet has direct chordal attachments to the septum without the presence of a developed papillary muscle in most cases.
Several structures of major surgical importance surround the TV, such as the right coronary artery (which is very often in close contiguity with the parietal portion of the tricuspid annulus), the atrioventricular (AV) node, the bundle of His and the coronary sinus (Figure 4).
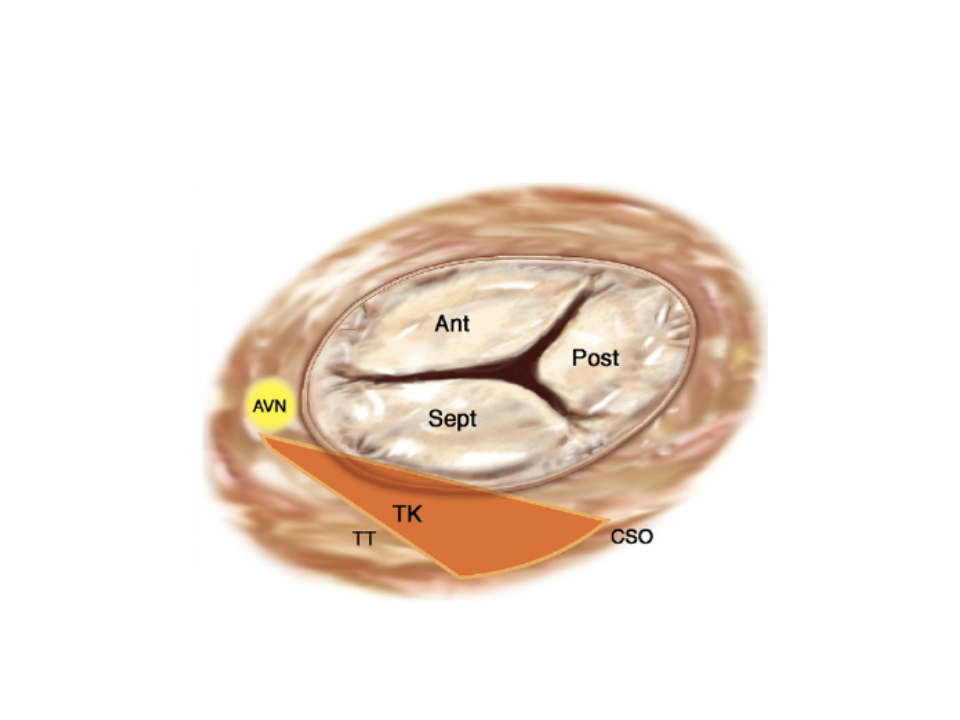
Surgical anatomy of the tricuspid valve.
The triangle of Koch (TK) is indicated by the orange area.
Ant: anterior; AVN: atrioventricular node; CSO: coronary sinus orifice; Post: posterior; Sept: septal; TT: tendon of Todaro
The big non-planar and non-circular structure of the tricuspid annulus must be taken into account when considering tricuspid technologies which anchors to the annulus. The normal shape of the tricuspid annulus is semi-lunar and it is larger compared to the mitral one. Under physiological conditions, the tricuspid annulus has a non-planar three-dimensional saddle-shaped configuration. Such a characteristic is lost in cases of functional TR, since the annulus becomes larger and flat. In the presence of functional TR, as a consequence of RV enlargement and dysfunction, the tricuspid annulus becomes more circular as it dilates in its anterior-posterior aspect.
Echocardiography is the essential imaging technique in the assessment of patients with TR.
Two-dimensional (2D) echocardiography is the imaging technique of choice to diagnose the mechanism and severity of TR. Echocardiography is also of help to define TV morphology and RV anatomy and function. In addition, three-dimensional (3D) is increasingly recognized as an important adjunct to 2D TTE for a more comprehensive assessment of the complex morphology of the TV.
The main echographic views used to assess leaflet anatomy of the TV with 2D TTE are the parasternal long-axis view of RV inflow, the parasternal short-axis view at the level of the aortic valve, and the apical four-chamber and subcostal views. Due to the complex morphology of the TV, only two leaflets can generally be visualized at a time, with some uncertainty regarding their precise identification, according to the various cutting planes. Commissures are difficult to assess with 2D techniques, unless a short-axis view of the TV can be obtained (low parasternal and subcostal short-axis view) , , . Conversely, real-time 3D TTE, when available, may provide an “en face” view of the TV which allows visualisation of the three leaflets simultaneously, precise identification of the commissures and assessment of leaflet coaptation .
Using TTE, the tricuspid annular diameter is generally measured from the apical four-chamber view, at end-diastole. The normal annulus diameter in adults is 28±5 mm and significant tricuspid annular dilatation is defined by a diastolic diameter ≥40 mm or >21 mm/m2 measured at end-diastole in the apical four-chamber view . However, measurement of the tricuspid annulus diameter with 2D as compared to 3D echocardiography techniques systematically underestimates the actual tricuspid annulus diameter . Although not routinely used, 3D echocardiography off-line multiplane reconstructions allow accurate measurement of the tricuspid annular dimensions .
The approach to assessing TR includes the identification of the type and mechanism of regurgitation, grading of the severity of TR, quantitative assessment of annular dilatation and tethering, assessment of RV dimensions and function, estimation of pulmonary artery pressure and assessment of inferior vena cava (IVC) and suprahepatic vein dimensions .
The assessment of type and mechanism of TR requires a careful assessment of leaflet morphology and mobility and annular morphology. Mechanisms of primary TR may involve prolapse of one or more leaflets in degenerative TR, restricted motion of the leaflets as the consequence of rheumatic disease or carcinoid or toxic disease or more rarely leaflet perforation due to endocarditis, post-traumatic disease, or congenital disease such as Ebstein’s anomaly.
In functional TR, it is important to determine the relative role of annular dilatation and tethering of the leaflets, as well as to clarify the potential role of additional mechanisms (e.g., interference with pacemaker electrodes).
Significant tricuspid annular dilatation is defined by a diameter ≥40 mm or >21 mm/m2 measured at end-diastole in the apical four-chamber transthoracic view. In functional TR, the degree of tethering of the leaflets can be evaluated by the measurement of the systolic tenting area (area between the tricuspid annulus and the body of the tricuspid leaflets) and the coaptation distance (distance between the tricuspid annular plane and the point of coaptation) in mid-systole from the apical four-chamber view. Severe tethering is defined by a tenting area >1 cm2 and coaptation distance ≥8 mm , .
Accurate grading of TR remains difficult due to the high dependency of TR to loading conditions, and the lack of objective and reproducible parameters , as for mitral regurgitation. Therefore, an integrative multimodality approach including qualitative and quantitative parameters is recommended. Colour Doppler flow provides a qualitative assessment of TR severity. The presence of a large central or eccentric jet reaching the posterior wall of the right atrium is in favour of severe TR. A dense continuous wave Doppler envelope with a triangular contour and early peak velocity, or a laminar TR flow also suggests severe TR. A more quantitative assessment is provided by vena contracta (VC) width measured in the parasternal or the apical four-chamber view. A VC ≥7 mm indicates severe TR, whereas a diameter <6 mm may be either mild or moderate TR. The assessment of proximal isovelocity surface area (PISA) of TR jet by the flow convergence method is not well validated for TR, because of the variable and non-circular morphology of the regurgitant orifice. The published data suggest that an effective regurgitant orifice area (EROA) >40 mm2 or a regurgitant volume >45 ml is indicative of severe TR. A more qualitative approach is to use the PISA radius at a constant aliasing velocity. A TR PISA radius ≥9 mm at a Nyquist limit of 28 cm/s suggests severe TR, whereas a radius <5 mm suggests mild TR . The presence of systolic hepatic flow reversal is specific to severe TR and represents the strongest additional parameter for evaluating the severity of TR.
Recently, with the advent of transcatheter technologies treating extreme patients, a new TR classification has been proposed to better characterize the anatomy and the severity of TR, including massive and torrential on top of the previous severity grades (Table 1) . Currently, no large data are available about these subgroups yet, but this new classification will have a significant impact on the design of future device trials and determine outcomes using quantitative echocardiographic parameters.
.jpg)
Proposed classification of TR grade beyond severe.
Adapted from Hahn et al. , with permission.
Due to the complex shape of the RV, assessment of RV enlargement requires measurement of RV dimensions in multiple views . RV systolic function can be assessed by multiple parameters: fractional area change (FAC), tricuspid annular plane systolic excursion (TAPSE) or tricuspid annulus systolic velocity. Of note, all these parameters have limitations in the presence of severe TR due to their dependence on loading conditions. Despite these limitations, TAPSE <16 mm and tricuspid annulus systolic velocity <11 cm/s could be used to identify patients with RV dysfunction , . Systolic pulmonary artery pressure can be estimated from the TR maximal velocity with an estimate of right atrial pressure on the basis of IVC size and collapse with respiration. Estimation of systolic pulmonary artery pressure is no longer possible in the case of laminar TR flow. IVC diameter and respiratory variations should be assessed from a subcostal approach, as well as systolic reversal in the central hepatic vein. The diameter of the IVC is an important parameter for assessing the degree of cardiovascular compensation, as well as for planning heterotopic implantation of transcatheter valves or stent implantation in the IVC. The diameter is measured perpendicularly to the long axis of the IVC at end-expiration, just proximal to the junction of the first hepatic vein which lies approximately 0.5 to 3.0 cm proximal to the ostium of the right atrium. The echocardiographic examination should also look carefully at the potentially associated valvular lesions, particularly on the left side, and assess LV systolic and diastolic function.
Both 2D and 3D TOE play an essential role in addition to fluoroscopy for procedural guidance during transcatheter TV interventions. The tricuspid valve is positioned anteriorly in the thorax far away from the oesophagus, with the mitral and aortic valve positioned in between. For this reason intraprocedural TOE is frequently more challenging compared to mitral procedures, especially when previous aortic/mitral prostheses are present. The availability of 3D TOE, biplane (xPlane) imaging and intracardiac echocardiography (ICE) is particularly helpful during these procedures. In patients with sub-optimal echocardiographic windows, the combined use of transthoracic echocardiography and these advanced imaging techniques may thus be of great utility.
Because of the complexity of TV anatomy and the need to improve communication and guidance during intervention, it is crucial that both interventionist and echocardiographer define before the procedure a limited number of imaging views with conventional orientation and anatomical landmarks. Immediate pre-procedural 2D TOE imaging allows comprehensive visualization of the TV leaflets in the different planes, measurement of TV mediolateral annulus and assessment of TR with colour Doppler (Figure 5). During the procedure, both 2D and 3D TOE are useful for precise guidance and positioning of devices, as well as for avoidance of complications such as coronary or chamber perforation. A 3D TOE “en face” view of the TV from the right atrial perspective provides identification of TV leaflets and localization of the anteroseptal and anteroposterior commissures (Figure 6). Care should be taken to include in the 3D data set the important anatomical landmarks that are the aorta and the interatrial septum. Despite a lower quality of 3D imaging for the TV as compared to those obtained for the mitral valve, a 3D “en face” view may help to position the devices during direct tricuspid valve annuloplasty techniques or for the clipping of the tricuspid leaflet. For tricuspid edge-to-edge repair specifically, the transgastric view provides the best “en face” view to perform device positioning, alignment and the grasping of the leaflets. Indeed, the pre-operative assessment of transgastric window is a key step of patient selection for TEER and patients with poor transgastric imaging are poor candidates for these procedures.
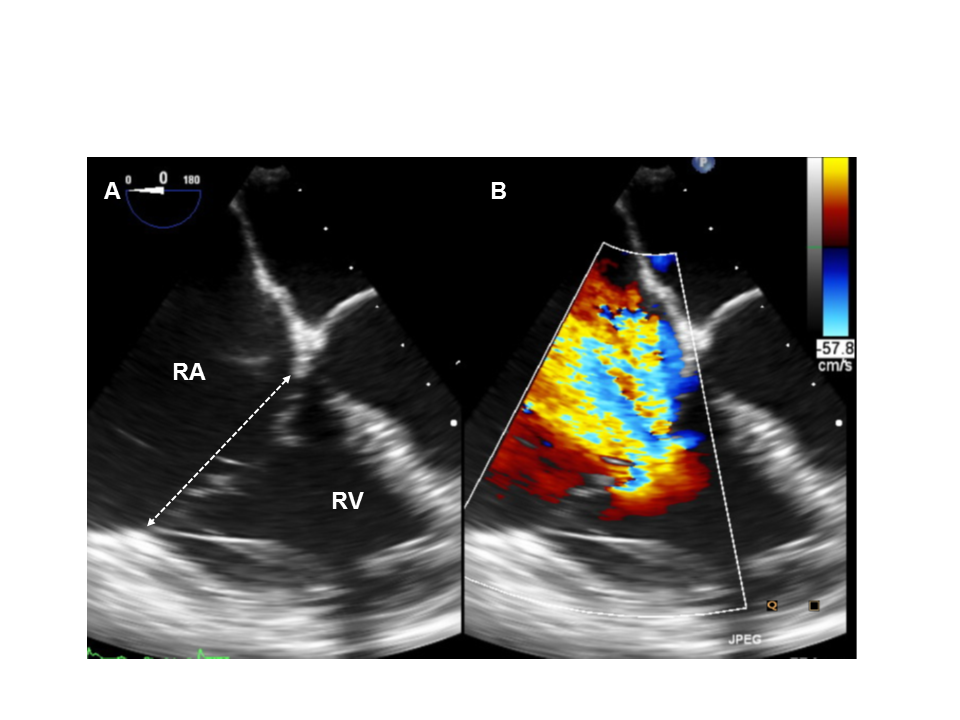
Pre-procedural transoesophageal echocardiography (TOE) assessment of septolateral annular dimension (A) and tricuspid regurgitation (TR) (B).
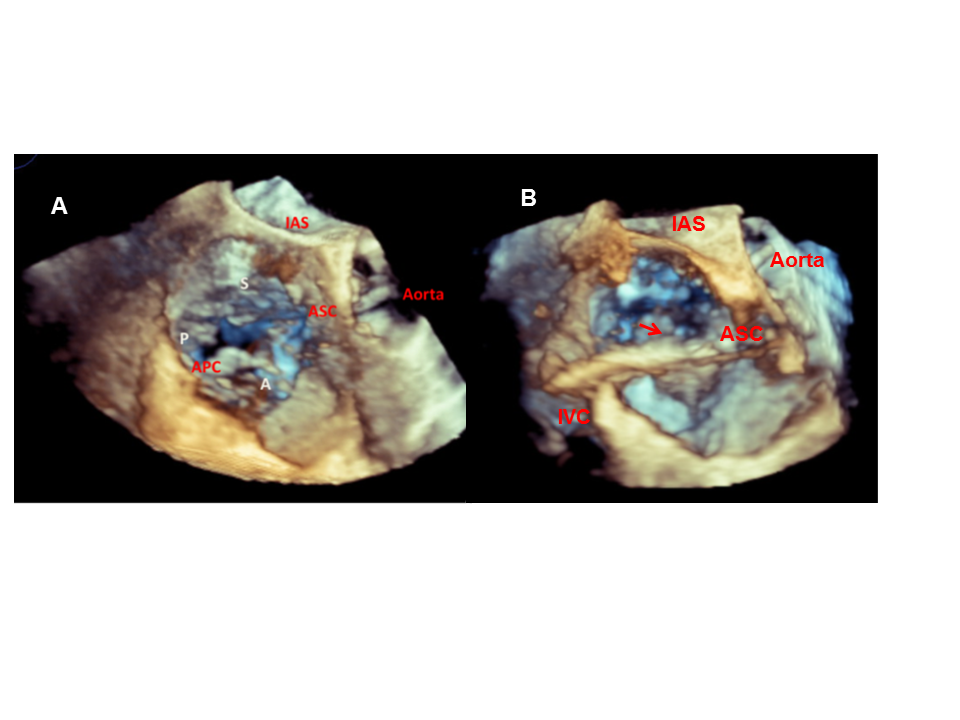
3D TOE en face view of the tricuspid valve (TV).
Identification of TV leaflets (A: anterior, S: septal, P: posterior) and localisation of the anteroseptal (AS) and anteroposterior (AP) commissures (A). Visualisation of the positioning of the TriCinch device (arrow) in the ASC (arrow) (B).
xPlane (or biplane) 2D TOE imaging provides simultaneous assessment of two orthogonal views. This mode is complementary to 3D TOE “en face” views and provides accurate identification of the TV leaflet insertion in the annulus, assessment of the course of the right coronary artery in relation to the AV groove, and good overall visualization of cardiac structures in real time (Figure 7).
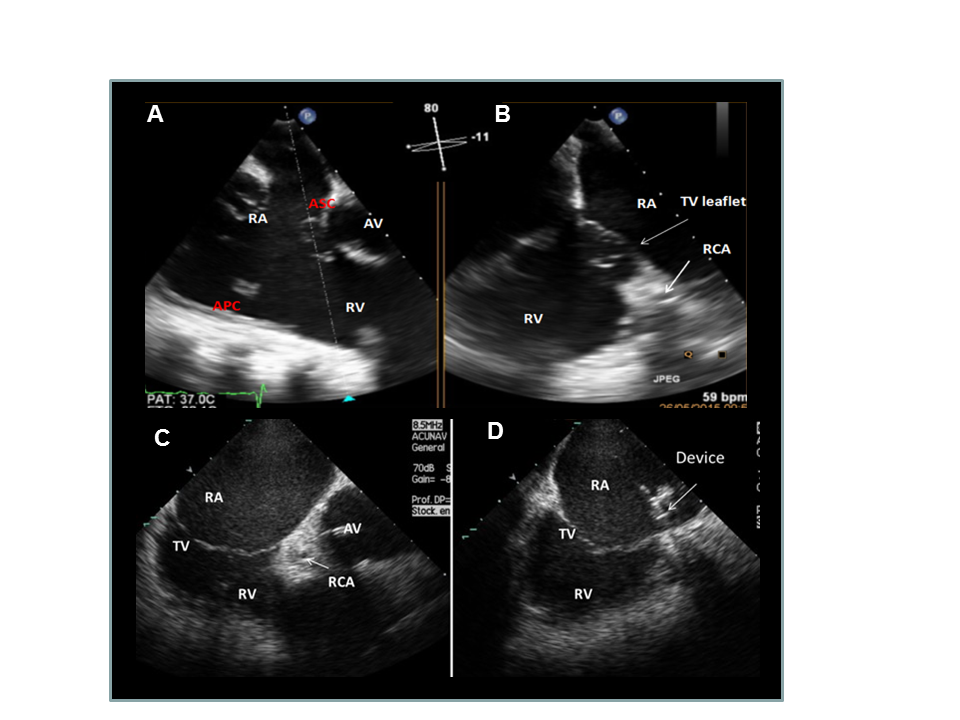
xPlane 2D TOE imaging (A and B) and ICE imaging (C and D) during intervention.
Identification of the target area on the anteroseptal (AS) commissure (A, B and C). Positioning of the catheter tip using ICE imaging (D).
APC: anteroposterior commissure; ASC: anteroseptal commissure; AV: aortic valve; RA: right atrium; RCA: coronary artery; RV: right ventricle
Because visualization of the tricuspid annulus may be difficult with 2D TOE only due to shadowing from prosthetic material, ICE imaging can be used simultaneously with TOE, to overcome this limitation. ICE imaging from the right atrium provides excellent visualization of the IVC-right atrial junction, tricuspid annulus and relative position of the right coronary artery to the insertion of the TV leaflet (Figure 6). In addition, the recent 4D volume ICE system (Siemens; Mountain View, California) provides even more accurate images and might find a wide application in this context. Cases of tricuspid clipping under ICE guidance in patients with suboptimal TEE imaging, have been described .
CT is an essential complementary tool to echocardiography for the pre-procedural planning of several tricuspid devices , especially those which target the tricuspid annulus for anchoring and those which may interfere with surrounding structures.
Since it offers a comprehensive assessment of the real 3D anatomy, CT allows the operator to assess the TV anatomy as well as the tricuspid annular shape and dimensions. Multiplanar reconstructions are performed to visualize all three TV leaflets, which are difficult to assess on the same short-axis “en face” view plane because of the non-planar morphology of the TV. The septal leaflet is best seen in the four-chamber view, and a two-chamber view of the right ventricle correctly shows the relationship of the posterior and anterior leaflets. Moreover, the two-chamber view is particularly appropriate for assessing the cavo-tricuspid isthmus superior to the hinge of the posterior TV leaflet, which is more difficult to visualize with TTE. Another major advantage of CT is the ability to combine both an anatomical evaluation of the tricuspid annulus and a semi-quantitative assessment of the tissue characteristics in the right atrioventricular groove. This is based on the measurements of the tissue density (in Hounsfield units) in studied regions of interest (allowing differentiation of calcification, epicardial fat, myocardium, fibrous tissue). This information regarding the structure of the TV annulus is of importance for the appreciation of the tissue quality that supports the leaflets. In case of TV annuloplasty rings, CT is the technique of choice for helping to identify any dislodgement, deformation in the shape, or inappropriate position. Finally, CT is capable of providing data in the adjacent structures including the coronary arteries, the systemic venous drainage and the hepatic veins. In certain pre-procedural evaluations, there is a need to identify the target zone of the implant, to assess the angle between IVC, SVC and the TV plane, to assess the distance between the right coronary artery and the valve annulus and assess the risk of injury of surrounding structures. It is also sometimes necessary to obtain detailed measurements of IVC and SVC dimensions, cavo-tricuspid isthmus length and hepatic veins (Figure 8, Figure 9).
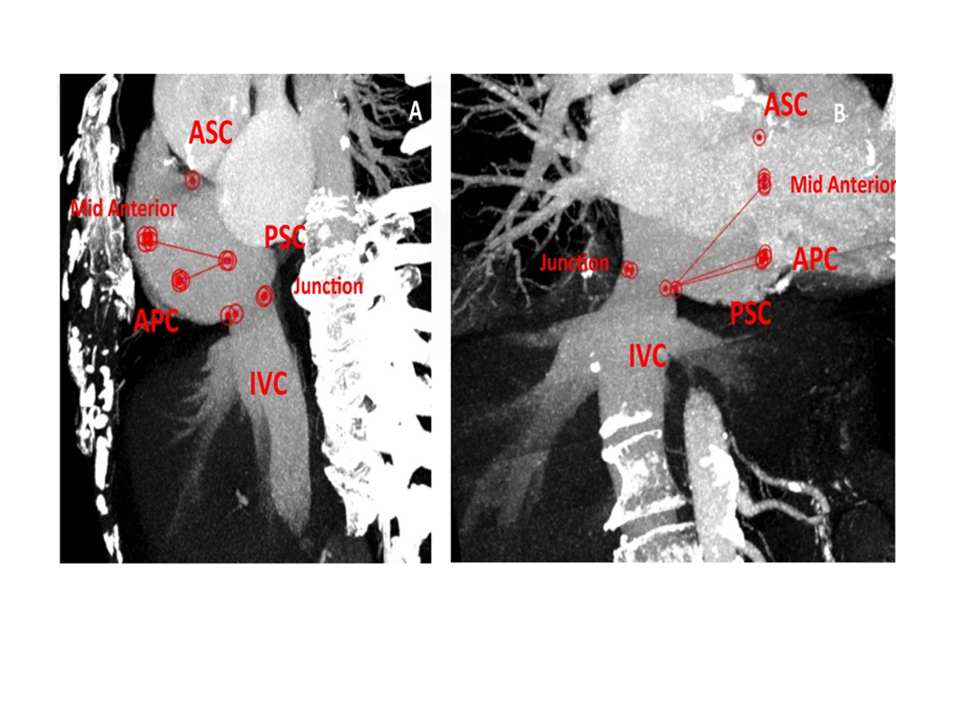
Tricuspid annulus distances and their relationship with IVC junction at the preoperative angio CT scan.
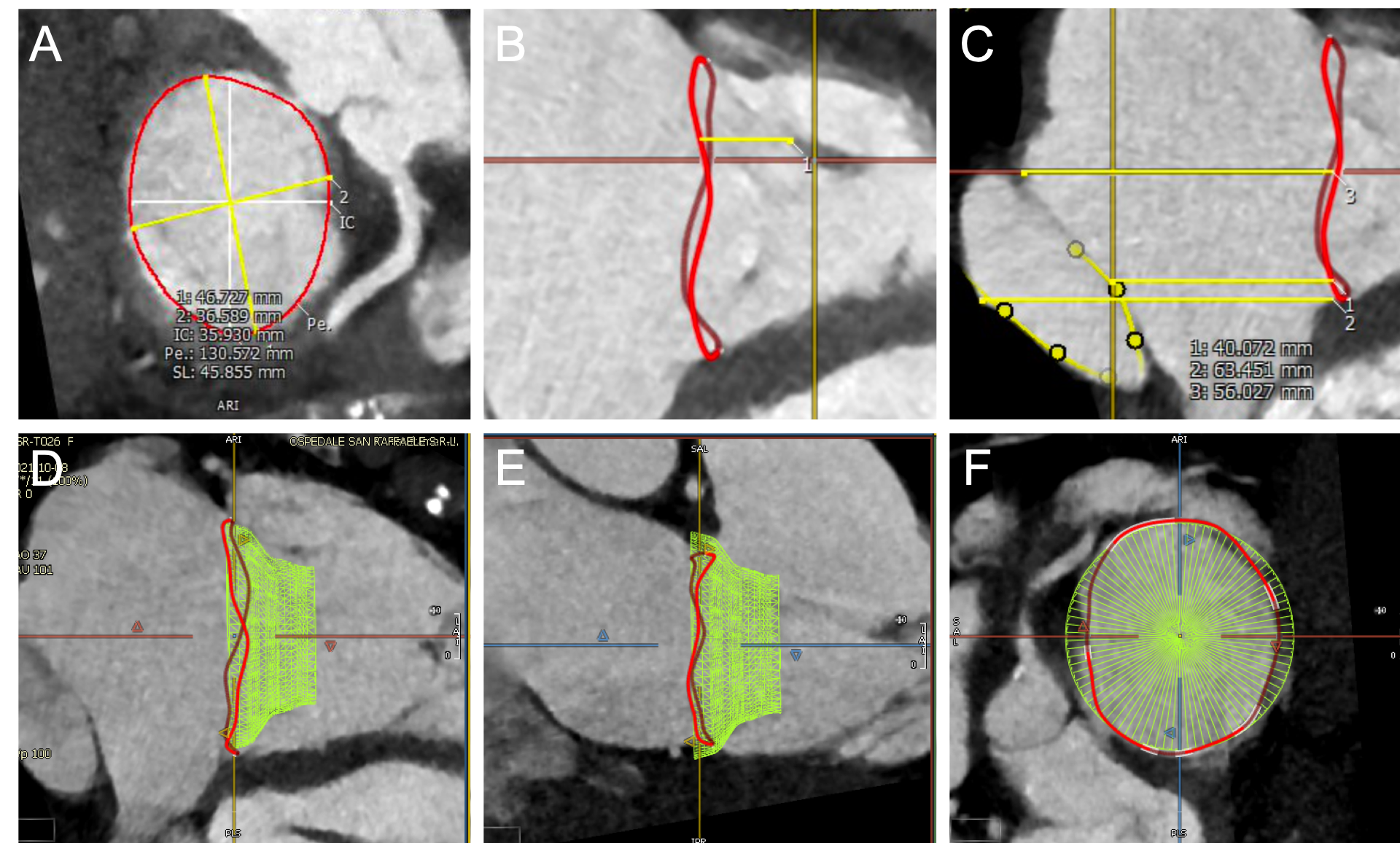
CT pre-procedural planning of transcatheter tricuspid valve implantation.
An example of Cardiovalve screening process is depicted. After segmentation of tricuspid annulus, the dimensions are measured (A). Further analyses are then carried out such as the distance between the annulus and the papillary muscles (B) and the relationship with the inferior vena cava (C). Finally, simulation of device positioning within the native annulus is performed (D,E,F).
Certain prerequisites must be fulfilled to obtain technically satisfactory images. Indeed, compared with the mitral valve which can be easily seen in routine cardiac CT, visualization of the right atrioventricular junction may be difficult and imaging of the tricuspid annulus and leaflets may be challenging. Depiction in detail of this region requires a good and homogeneous enhancement of the structures around the TV annulus, and elimination of any artefact is crucial. The major limitations are the right atrial artefact and superior vena cava artefact, due to the inhomogeneous enhancement of the right atrium and the high-attenuation effect of the contrast medium, respectively. Thus, cardiac CT protocols need modification for the right heart examination, especially with contrast agent administration. These artefacts can be attenuated when injections are performed through the peripheral veins of the lower limb.
Many different transcatheter approaches have already been developed to treat TR over the past decade. The Trivalve Registry, including more than 400 patients, provides a faithful description of early real-world adoption of such devices . Edge-to-edge leaflet repair was the most frequent procedure. Overall, transcatheter tricuspid correction showed a good safety (30-day mortality 3.6%), at the price of imperfect reduction of TR (TR£2 72.8%). The registry only gives insights on the very early phase of these technologies, some of them being used only in very few cases. Interestingly, a propensity matched analysis compared the survival of Trivalve patients who received TR correction to the survival of medically managed TR patients showed a clear survival benefit in the Trivalve group (1-year mortality 23 ±3% vs 36 ±3%, p=0.001) as well as reduced heart-failure events, also after adjusting for confounding factors . This result is very promising and opens the way to further RCTs.
The technologies/procedures with most current significance in clinical practice are briefly discussed below, Figure 10. While each of these devices is commonly used as a stand-alone intervention, some of them can be combined during the same procedure or added as a subsequent step to a previous operation.
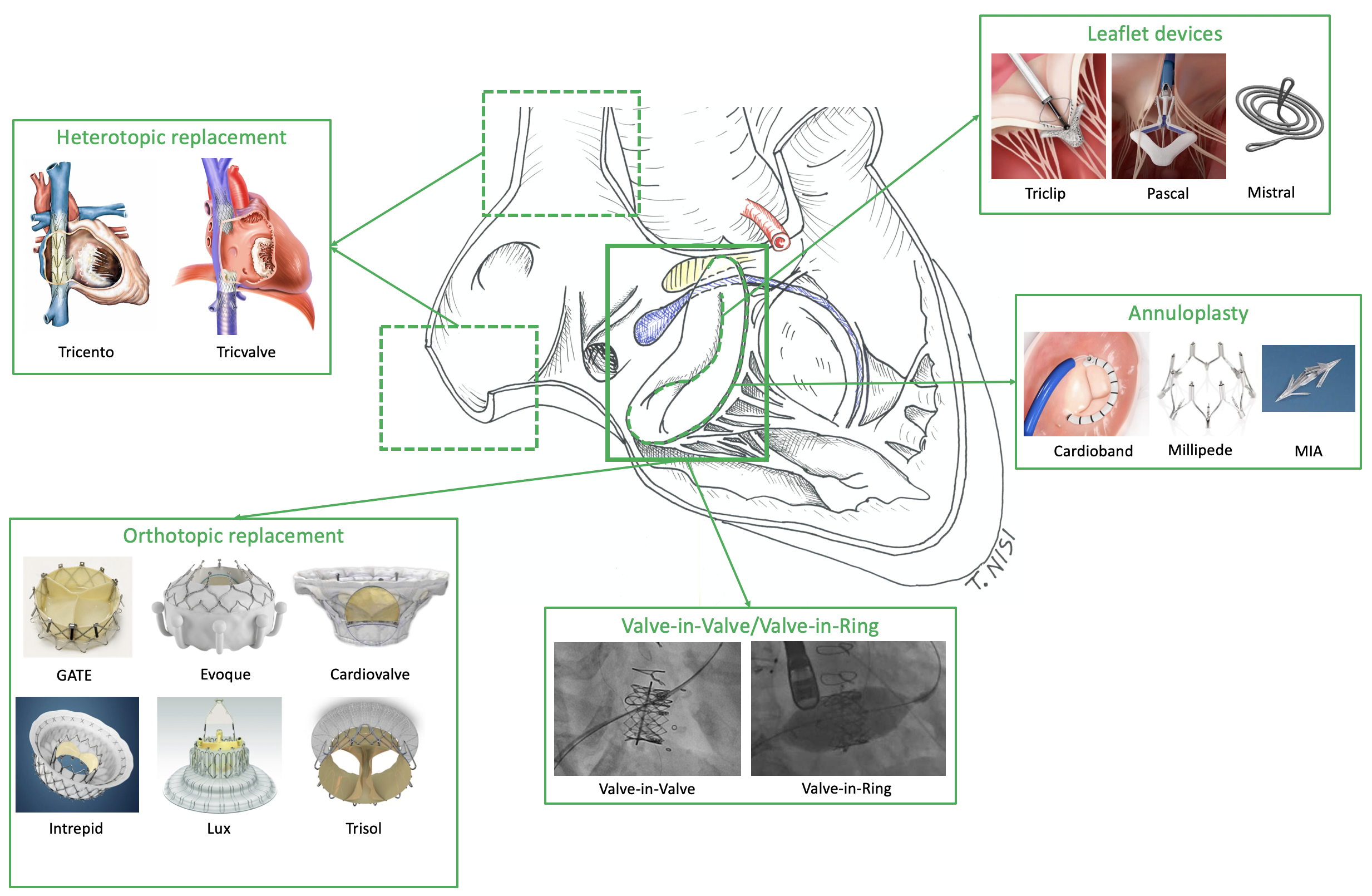
Landscape of currently most important transcatheter tricuspid devices.
Among the tricuspid surrounding structures, the conduction system is highlightened in blue, the membranous septum in yellow and the right coronary artery in red.
As for the mitral position, tricuspid transcatheter implantation of a transcatheter aortic valve (TAVI) prosthesis is a possible therapeutic option for patients with recurrent tricuspid pathology following a surgical implant. Percutaneous valve-in-valve and valve-in-ring implantation has been the subject of numerous reports, either for a degenerated tricuspid bioprosthesis or for ring annuloplasty failure .
Valve-in-Valve procedures are technically simple and quick. They are conducted through transfemoral route, using local anaesthesia and fluoroscopic guidance, without rapid pacing. Due to lower closing pressures, oversizing of the TAVI prosthesis is considered to be less crucial in the tricuspid position. If the patient is adequately selected in terms of preserved right ventricle function, the procedure provides excellent acute and midterm outcomes , .
Valve-in-ring procedures are more challenging, due to the open configuration, non-circular non-planar shape and rigidity of surgical tricuspid rings. These features are associated with sub-optimal results (device underexpansion and paravalvular leak) and therefore tricuspid valve-in-ring procedures should be considered with caution. On the other hand, unlike the mitral position, the risk of right ventricular outflow obstruction is absent . An integrated approach for the accurate sizing of the ring is mandatory using the three available techniques: CT, 3D TOE and fluoroscopy (Figure 11).
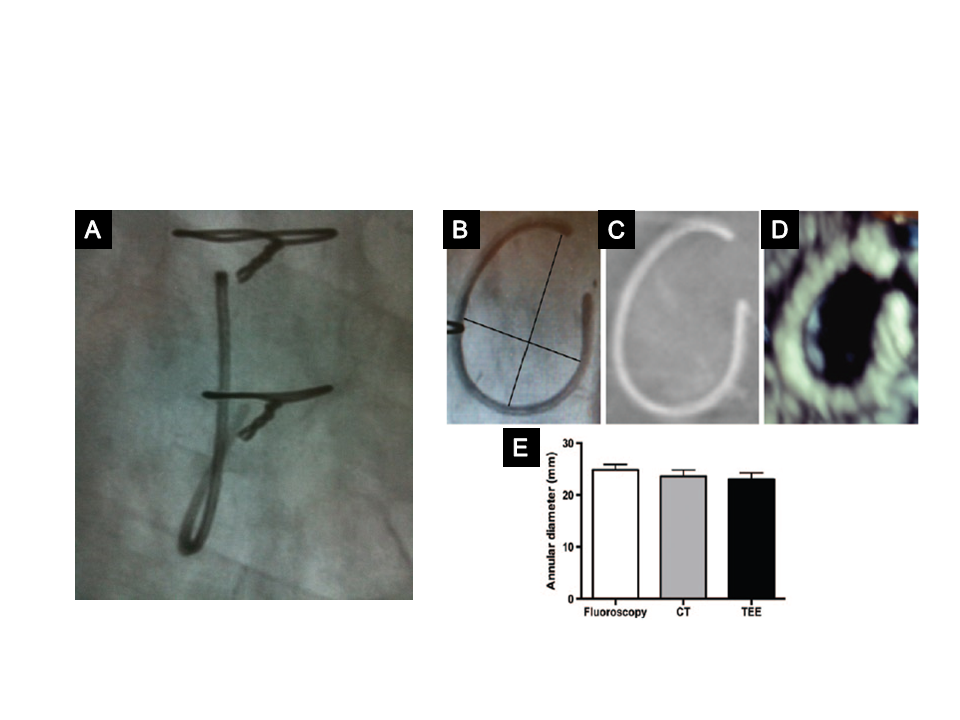
Typical tricuspid annuloplasty ring.
A) Fluoroscopic view of a tricuspid ring showing that it is non-circular, open and non-planar. B) - D) Measurement of the inner diameter of an annuloplasty ring, showing the long and short axis (black lines) taken into account to measure the inner diameter (B), 3D TOE (C), and CT scan (D). Mean inner diameter measurements using the three methods (E).
Edge-to-edge repair with the MitraClip system (Abbott) for mitral regurgitation is a validated technique . The same approach is used for TR. While the first tricuspid cases were done with the mitral device , a dedicated tricuspid technology is currently available to address the specific angles of the right heart, together with four different clip sizes (Figure 12). Thanks to the versatility and simplicity of the edge-to-edge concept, as well as the safety and refinement the technology, the Triclip is currently the most used percutaneous device for correction of TR worldwide.
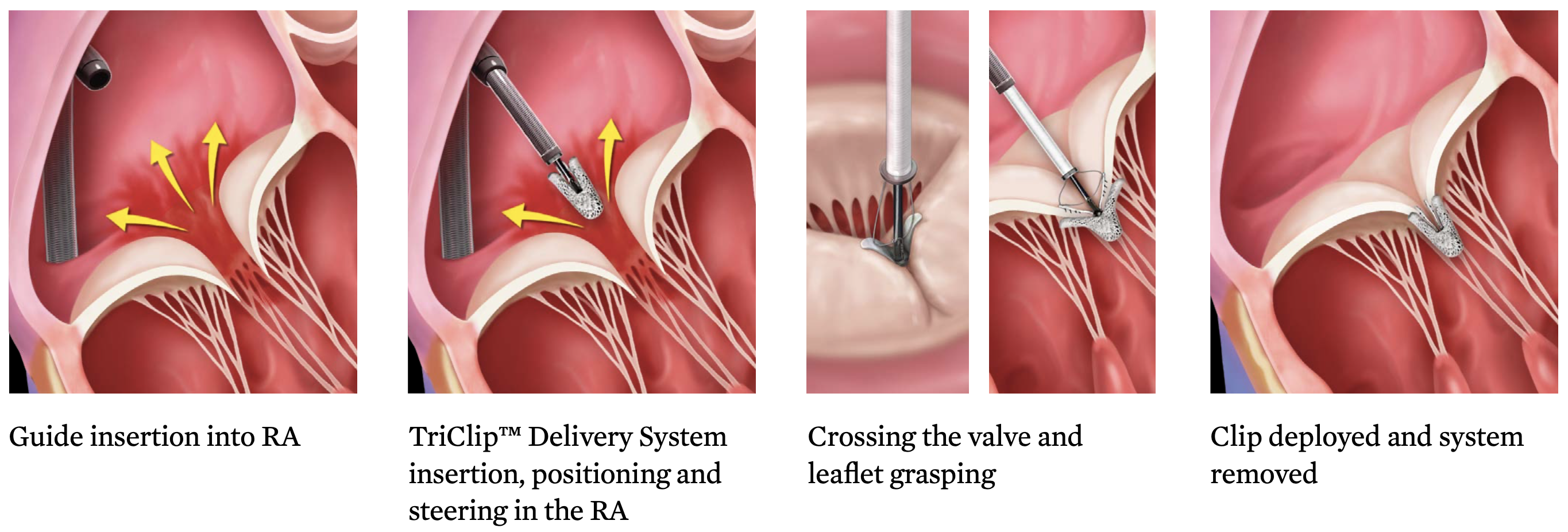
The Triclip device is the most used transcatheter tricuspid therapy in the world.
Clip arms and grippers capture the tricuspid leaflets, closure of the clip force leaflet coaptation. Four different clip sizes are currently available.
Similar to the mitral setting, the Triclip grasps the leaflets and approximates their free margin to force coaptation. Every part of the valve can be targeted but most frequently the septal and anterior leaflet are first joined, moving centrally with subsequent devices. Usually more than 1 clip is needed to achieve a significant TR reduction. Some degree of annular reduction can be observed sometimes.
The presence of a PM lead is not a contraindication to the procedure, although the location and interference with TR mechanism must be assessed for each specific case. Excessive lack of leaflet coaptation (>8.5mm) , and poor imaging quality represent the most important contraindications to the Triclip procedure.
Procedural safety is excellent, the Triluminate trial reporting no periprocedural death nor complication. At 1-year all-cause mortality was 7.1%, TR was reduced to moderate or less in 71% of patients with significant improvement in symptoms and quality of life . The Triluminate Pivotal RCT (NCT03904147) currently ongoing will compare Triclip to medical therapy in respect to survival, tricuspid surgery, heart-failure hospitalization and quality of life.
The Pascal tricuspid valve repair system (Edwards Lifesciences, Irvine, California) is a 22 Fr system that combines the advantages of leaflet grasping to the physical properties of a spacer to overcome some limitations observed in cases of large coaptation gaps and to further reduce the total regurgitant area. It is currently available also with a narrower conformation without central spacer (Pascal ACE).
Several studies confirmed efficacy and safety. The most recent US experience on 34 patients reported no procedural mortality, a 30-day adverse event rate of 5.9% and 52% of patients with residuao TR moderate or less . The device obtained CE approval in 2020.
Another promising technology is represented by the Mistral TVRS (Mitralix, Ltd.). It works increasing leaflet coaptation and is made of an 8.5 Fr delivery system and a spiral-shaped repair device creating a “bouquet” of chordae tendinae. Of note, this technology would not preclude the use of annuloplasty or leaflet technology at a later stage. Early results on 7 patients demonstrated safety and efficacy of such innovative device .
The Cardioband (Edwards Lifesciences, Irvine, California) is a transcatheter transfemoral direct annuloplasty device that closely reproduces surgical annuloplasty with a ring. It is implanted on the atrial side of the tricuspidal annulus from commissure to commissure, with the beating heart under fluoroscopic and echocardiographic guidance.
The Cardioband is made of a polyester sleeve available in six lengths to cover a wide range of annulus circumference sizes. During implantation, 12 to 17 metal anchors are inserted to fixate the Cardioband to the tissue. Each anchor is 6 mm long and is spiral-shaped with a screw-like fixating mechanism designed to secure the implant safely in place. All anchors are repositionable and retrievable until disconnected from the anchor drive. Once the Cardioband is successfully anchored along the annulus, the band is dynamically cinched using a dedicated tether achieving a reduction of the tricuspid annular diameter of approximately 30%.
Two-years data from the single-arm, multicenter, prospective TRI-REPAIR study were recently published . The study included 30 symptomatic patients. Technical success was achieved in all patients and only one in-hospital death was recorded. At 2-years follow-up, 8 deaths (27%) occurred. Functional and symptoms improvements were recorded over a two-years observation, with a significant reduction in septolateral annular diameter of 16% and 72% of patients with ≤moderate TR grade.
Several other systems to achieve tricuspid annular reduction are in an earlier phase of development, such as the Millepede (Boston Scientific) and the MIA technology (Micro Interventional Devices). The MIA is a novel sutureless annuloplasty system consisting of compliant, self- tensioning low-mass anchors and a thermoplastic elastomer (MyoLast) deployed in the tricuspid annulus. The feasibility and safety study (STTAR trial, not registered) is underway to assess this novel technology among 40 patients with FTR.
Transcatheter tricuspid valve replacement is slowly emerging. Some devices have been developed for the mitral valve and then transposed to the tricuspid, some other have been originally designed for the tricuspid. Compared to the mitral apparatus, the tricuspid valve brings peculiar features to be noted:
Only few highly selected patients, not treatable with other therapies, are currently submitted to transcatheter tricuspid valve implantation. Early results are promising but it is worth to be remembered that besides the anatomical-technical device selection the key point to achieve good outcomes for such patients is RV function. RV failure due to afterload mismatch following TR abolition after tricuspid replacement is a typical and dreadful complication of these patients and must be avoided through careful pre-procedural selection, just like in beating-heart open tricuspid surgery. Like in surgery, borderline cases with hard-to-define RV function may benefit before the procedure from i.v. diuretics and inotropes to optimize fluid balance and test the reserve contractility of the RV. This will help to identify patients who will survive the procedure but will also make them receive the procedure in the best possible condition.
The Gate (NaviGate Cardiac Structures, Inc.) atrioventricular valve is a biological valved stent and has been the first to be implanted in native orthotopic position to achieve tricuspid valve replacement; it consists of conical-shaped valve with Nitinol alloy stent into which is mounted a trileaflet valvular mechanism fabricated from pre-treated equine pericardium. The specific design serves the purpose of avoiding flow dynamics alterations and reducing right atrium and right ventricle protrusion. The valve anchoring is based on specially configured structures, winglets and graspers, 12 located in the proximal part of the stent and 12 in the distal end of the stent. It can be implanted transatrially through a minimally invasive right thoracotomy directly through the free wall of the RA into the TV. Alternatively, the transjugular route has been tested with a 45 Fr hydrophilic introducer sheath.
Data about 30 patients with severe or greater TR have been described: technical success was 87% and the most used access site was the transatrial approach. In hospital mortality was 10%, increasing to 13% at 30-day follow-up. Procedural complications included malpositioning requiring surgical conversion, pacemaker implantation due conduction abnormalities and RV perforation.
The Evoque system (Edwards Lifesciences) consists in a self-expanding nitinol frame covered in fabric that anchors to tricuspid annulus, leaflets and chords through several ventricular hooks. 1-year outcomes of compassionate use in 27 patients with at least severe TR have been published. At 1 year survival was 93%, NYHA I/II class was observed in 70% and TR £2+ was observed in 96% of patients . A RCT comparing the Evoque to optimal medical therapy in currently ongoing (NCT04482062).
The Cardiovalve presents a similar design, with 12 ventricular arms that grasp the tricuspid leaflets before an atrial flange sandwiches from above the leaflets and the annulus to provide sealing and stabilize the fixation.
The device is currently undergoing a clinical early feasibility study, more than 15 cases have been treated wordwide with promising results.
Several other new technologies are under investigation in an early phase, such as the Intrepid (Medtronic), the Lux-Valve (Jenscare Biotechnology), and Trisol Valve (TriSol Medical Ltd., Inc., Yokneam, Israel), which uniquely consists of a single bovine pericardial piece closing in a dome shaped structure with the aim of avoiding RV sudden afterload increase.
Heterotopic TV implantation was created to treat easily patients affected by TR before orthotopic transcatheter tricuspid replacement and repair were developed. In such approach transcatheter prostheses are implanted in the superior/inferior vena cava or in the right atrium, away from the native tricuspid valve. Currently these procedure are reserved as a palliative treatment to extreme symptomatic patients who have no other therapeutic option. The procedures are technically simple, the goal is to reduce the peripheral oedema. .
From a technical perspective, the large and variable diameters of the IVC and SVC and the length of the landing zone between the hepatic veins and inferior cavo–right atrial junction are major challenges. Another unsolved issue is the long-term tolerability of this palliative technique, especially regarding the risk of deterioration of RV function and the right atrium ventricularization.
Different types of transcatheter heart valves have been used in the past: balloon-expandable TAVI valves (SAPIEN valve, Edwards Lifesciences) and specifically developed self-expanding valves: TricValve (P&F Products Features Vertriebs) and Tricento (NVT) , . However, due to safety issues (embolization) TAVI valves are currently no more used (TRICAVAL trial, NCT02387697).
Among the dedicated devices, the Tricento is made of a long custom made stent connecting the two venae cavae with a valve in the middle at the level of the right atrium. The TricValve on the other hand is made up of two separate self-expandable valve to be implanted in the superior and inferior vena cava. These procedures remain rare and few evidences are available. In a recent series of 21 patients treated with the Tricento device, promising symptoms improvement and RV volume reduction after 2 months were observed .
Although surgery is the gold standard for the treatment of tricuspid endocarditis, a number of high-risk patients who were poor candidates for surgery have been recently treated with mechanical aspiration using the Angio-VAC system to remove bulkly vegetations in the setting of tricuspid endocarditis . While few evidences are available, this option looks very appealing for selected sick patients, especially when recurring.
Depending on the clinical status of the patient, the anatomical target, the pathophysiological mechanism of action and the available initial data, we can now start to clarify some general principles to help in the selection process of different transcatheter tricuspid devices, Table 2, Figure 13 . New evidences and developments to come will allow further refinements in the near future.
.jpg)
Anatomical criteria for transcatheter tricuspid device selection.
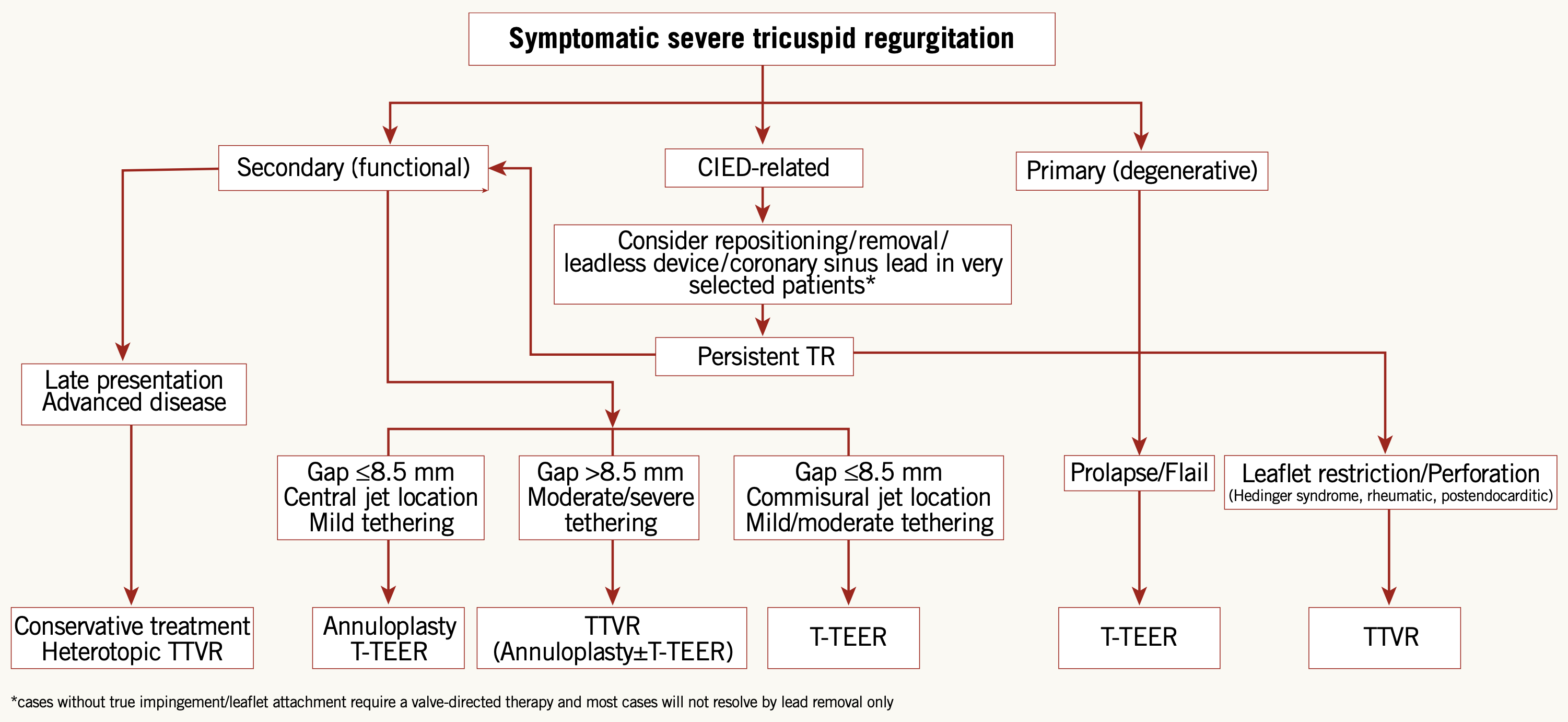
Transcatheter tricuspid device selection flowchart.
Reproduced with permission from Praz et al. .
In addition to pre-procedural echocardiographic and CT evaluation, right heart catheterization is the gold standard to assess pulmonary hypertension and pulmonary vascular resistance and represent a fundamental step of tricuspid patients assessment , especially before a tricuspid valve replacement procedure.
Tricuspid patients frequently present very sick due to both extracardiac comorbidities and impaired heart function. Especially in the setting of functional TR, the right ventricle is the true final determinant of patients outcome. Assessment of right ventricle function is challenging in the presence of severe TR. Some of these functional TR patients, especially those affected by long lasting disease, may benefit from a short “pre-habilitation” before the procedure. This is done optimizing not only cardiac preload through i.v. diuretics but also ventricular contractility with inotropes. This approach has multiple advantages: indeed the assessment of ventricular contractile reserve is per se important for patient selection, moreover the improved cardiac output will lead to improved liver and kidney function which all together will maximize patients ability to go safely through the procedure.
After the procedure, baseline diuretic dosage should initially be maintained for several weeks/months to favor ventricular reverse remodeling and repeated outpatient follow-up are recommended to adjust optimal medical therapy.
Tricuspid repair devices require the same antiplatelet regimen as transcatheter mitral repair (usually four weeks of aspirin plus clopidogrel, followed by aspirin only). Proper antithrombotic management for transcatheter tricuspid replacement is still unclear although early experience suggests that anticoagulation with warfarin, due to the bulky prosthetic design and the low flow in the right heart chambers, may be safer that antiplatelet therapy alone.
After being neglected for decades, the tricuspid valve is now under the spotlight. Tricuspid disease is frequent and impairs survival as well as quality of life. Surgery has provided unsatisfactory results in the past and requires strict selection and careful management to achieve good outcomes. Transcatheter technologies are still in their very early phase but they promise to allow more patients to be able to access treatment, thanks to their low invasiveness. Different devices are already available, targeting all possible disease mechanisms. Tricuspid repair with leaflet edge-to-edge approximation is currently the most developed technology and the most used.
Imaging, with echocardiography and CT, is more challenging compared to the aortic and mitral valves, but it is key for all the technologies to correctly select the anatomy and guide the procedure.
Especially in the setting of secondary TR, understanding the right ventricle function is fundamental: repair techniques achieve increased efficacy when treating less advanced ventricles while replacement is safer when performed in ventricles able to withstand acute afterload mismatch after abolition of TR.
When discussing the tricuspid valve we are only scratching the surface. Important lessons have already been learned from the surgical experience over the past 20 years and should not be neglected. On the other hand transcatheter technologies work in their own way, in the full live beating heart, and represent a unique opportunity to further expand the tricuspid knowledge. Several new gaps are to be filled in the next years to improve our understanding of tricuspid disease:
Horacio Medina de Chazal, Ali Zgheib, Angelo Quagliana, Michael Chetrit, Jean Buithieu, Giuseppe Martucci, Marco Spaziano, Ali Abualsaud, Ole de Baker, Laurence Campens, Pascal Theriault-Lauzier, Jere...
Updated on November 23, 2022
Horacio Medina de Chazal, Ali Zgheib, Angelo Quagliana, Michael Chetrit, Jean Buithieu, Giuseppe Martucci, Marco Spaziano, Ali Abualsaud, Ole de Baker, Laurence Campens, Pascal Theriault-Lauzier, Jere...
Updated on November 17, 2023