Horacio Medina de Chazal, Ali Zgheib, Angelo Quagliana, Michael Chetrit, Jean Buithieu, Giuseppe Martucci, Marco Spaziano, Ali Abualsaud, Ole de Baker, Laurence Campens, Pascal Theriault-Lauzier, Jere...
Updated on November 23, 2022
Aortic stenosis (AS) is the most common valvular heart disease leading to intervention. It is characterized by progression from leaflet thickening and calcification to significant haemodynamic stenosis which results in disease-specific symptoms and physical limitations as well as poor prognosis and impaired quality of life if left untreated.
In 1986, Cribier and colleagues introduced balloon aortic valvuloplasty as treatment for inoperable patients with severe AS. Although balloon aortic valvuloplasty achieved favourable acute haemodynamic outcomes, restenosis and clinical deterioration occurred frequently within 6-12 months requiring repeat interventions, . In 2002, Cribier and colleagues performed the first in-human transcatheter aortic valve implantation (TAVI) using a 24 Fr catheter delivery system housing a 23 mm bovine pericardial balloon-expandable stent valve in a 57-year-old critically ill patient presenting with cardiogenic shock due to severe AS who had failed balloon valvuloplasty (Figure 1). The intervention was successful in restoring stable haemodynamics leading to subsequent feasibility studies with the balloon-expandable transcatheter heart valve (THV), , . In parallel, Grube and colleagues reported the first-in-human results of a self-expanding THV consisting of a nitinol frame and porcine pericardial leaflets (Figure 2), . The early feasibility studies with balloon-expandable or self-expanding THV consistently demonstrated procedural success resulting in significant haemodynamic improvement with favourable short-term clinical outcomes (Table 1), , , , , ushering in a new era in the management of patients with severe AS.
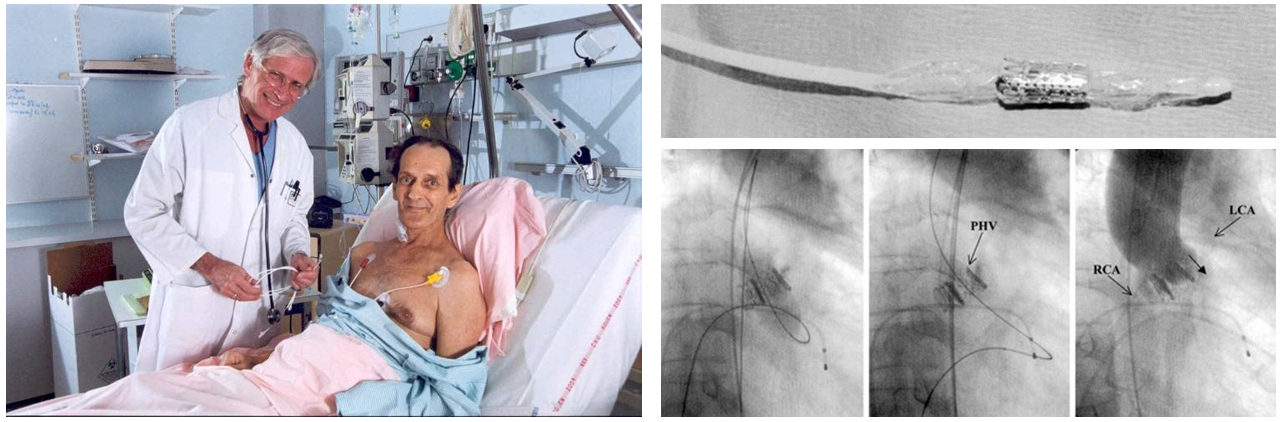
First-in-human transcatheter aortic valve implantation.
Figures reproduced and modified from Cribier et al. Circulation 2002;106(24):3006-3008 and PCR Online (https://www.pcronline.com).
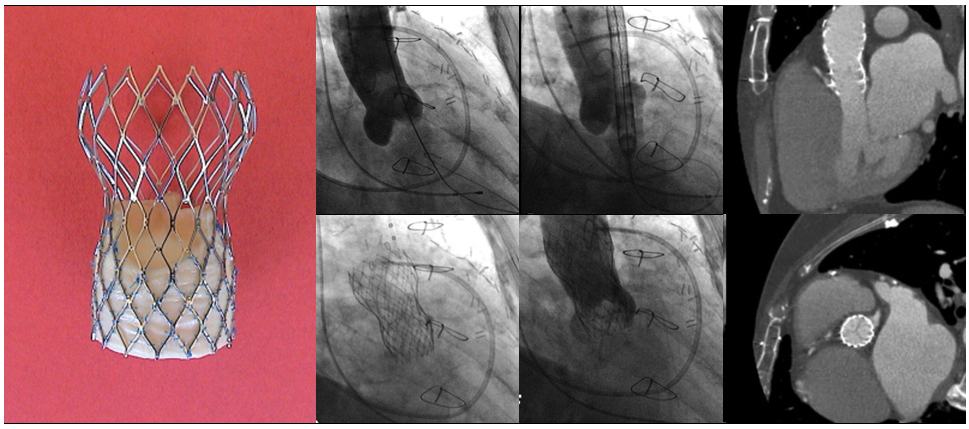
First implantation of the self-expanding CoreValve System.
Figures reproduced and modified from Grube et al. Catheter Cardiovasc Interv 2005;66(4):465-9.
Following the feasibility studies, TAVI has been directly compared with surgical aortic valve replacement (SAVR) in a series of randomized control trials (RCTs) across the spectrum of surgical risk, demonstrating favourable clinical outcomes, , , , , , , , , , , , . Following regulatory approval in Europe in 2007 and in the United States in 2011, TAVI has been widely adopted and continues to grow exponentially (Figure 3), , . In this chapter, we will provide a detailed description of current indications, patient selection for TAVI, and the procedural considerations. Furthermore, we will summarize the available evidence and emerging indications in the field of TAVI.
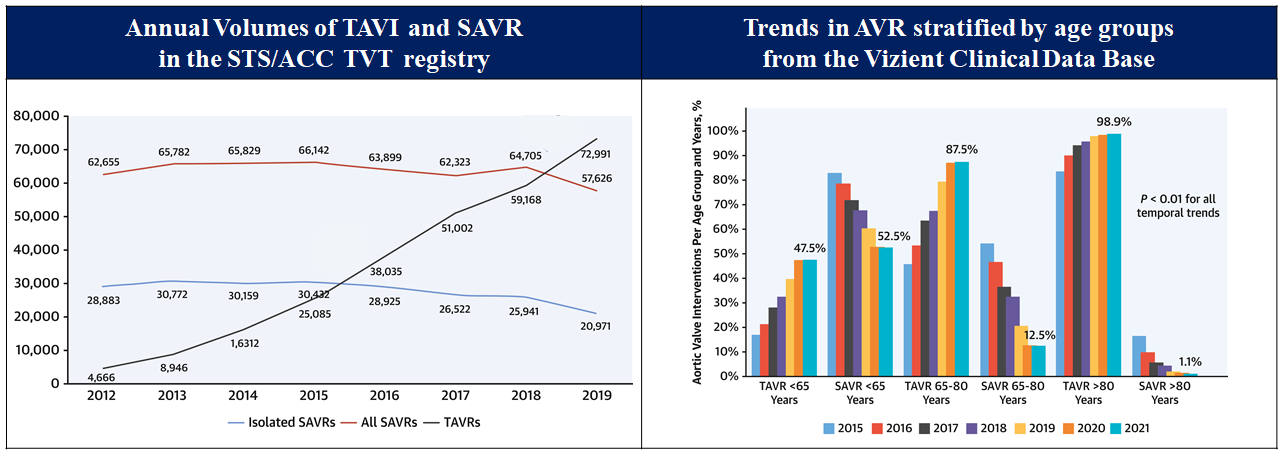
National trend in aortic valve implantation.
Figures reproduced and modified from Carroll et al. J Am Coll Cardiol 2020;76(21):2492-2516 and Sharma et al. J Am Coll Cardiol 222;80(21)2054-2056.
Table 1. Early feasibility studies of TAVI
| Study | N | Age (years) | Surgical risk score | Access site | AVA (cm²) |
mPG (mmHg) |
Procedural results | 30 days | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | Successful implantation, n (%) |
Death, n (%) | Death, n (%) | |||||||
| Balloon-expandable | Cribier et al. (2004) | 6 | 75 ± 12 | / | femoral vein | 0.5 ± 0.1 | 1.7 ± 0.1 | 38 ± 11 | 5.6 ± 3.4 | 5 (83) | 1 (17) | / | |
| Cribier et al. (2006) | 27 | 80 ± 7 | 12 ± 2a | femoral vein or artery | 0.6 ± 0.1 | 1.7 ± 0.1 | 37 ± 13 | 9 ± 2 | 21 (78) | 2 (74) | 6 (22) | ||
| Webb et al. (2006) | 18 | 81 ± 6 | 26.2 ± 13.1b | femoral artery | 0.6 ± 0.2 | 1.6 ± 0.4 | 50 ± 12 | 13 ± 6 | 18 (100) | 0 | 2 (11) | ||
| Walther et al. (2008) | 50 | 82 ± 5 | 27.6 ± 12.2 b | trans-apical | / | 7.2 ± 3.9 | 47 (94) | 0 | 47 (94) | / | / | ||
| Self-expanding | Grube et al. (2006) | 25 | 80 ± 5 | 10.97 (9.2-19.9) b | femoral artery | 0.7 ± 0.1 | / | 44.2 ± 10.8 | 12.4 ± 3 | 22 (88) | 2 (8) | 5 (20) | |
| Grube et al. (2007) | 86 | 82 ± 6 | 21.7 ± 12.6 b | femoral artery | 0.6 ± 0.2 | / | 43.7 ± 15.4 | significant decrease | 76 (88) | 5 (6) | 10 (12) | ||
|
a: Additive EuroSCORE. b: Logistic EuroSCORE. AVA = aortic valve area; mPG = mean transvalvular gradient. |
|||||||||||||
The global burden of calcific aortic valve disease continues to increase due to aging and population growth. In 2021, there were an estimated 13.3 million cases, predominantly observed in the elderly population, and an estimated 151,000 deaths and 2,140,000 disability-adjusted life years attributable to non-rheumatic calcified aortic valve disease (Figure 4 and Figure 5), . A pooled analysis of 11,911 adults from three large, national, population-based epidemiological studies, combined with data from 16,501 adults in Olmsted County, demonstrated an age-dependent increase in the prevalence of moderate or severe AS (in population-based studies: 0.02% in 18-44, 0.1% in 45-54, 0.2% in 55-64, 1.3% in 65-74, and 2.8% in ≥75 years; in Olmsted County: 0.1% in 18-44, 0.2% in 45-54, 0.6% in 55-64, 1.4% in 65-74, and 4.6% in ≥75 years, respectively). The actual incidence and prevalence of AS may be underestimated. The OxVALVE Population Cohort Study enrolled 2,500 adults aged ≥65 years from a primary care population and screened for undiagnosed valvular heart disease. In this study, AS was newly diagnosed in 1.3% of participants, and over half of these patients had moderate or severe AS. This study suggested a substantial increase in the clinical and economic consequences of clinically significant valvular heart disease within the rapidly expanding elderly population. Indeed, recent data from Olmsted County indicate that although the incidence of severe AS has remained stable, the absolute number of AS cases has increased.
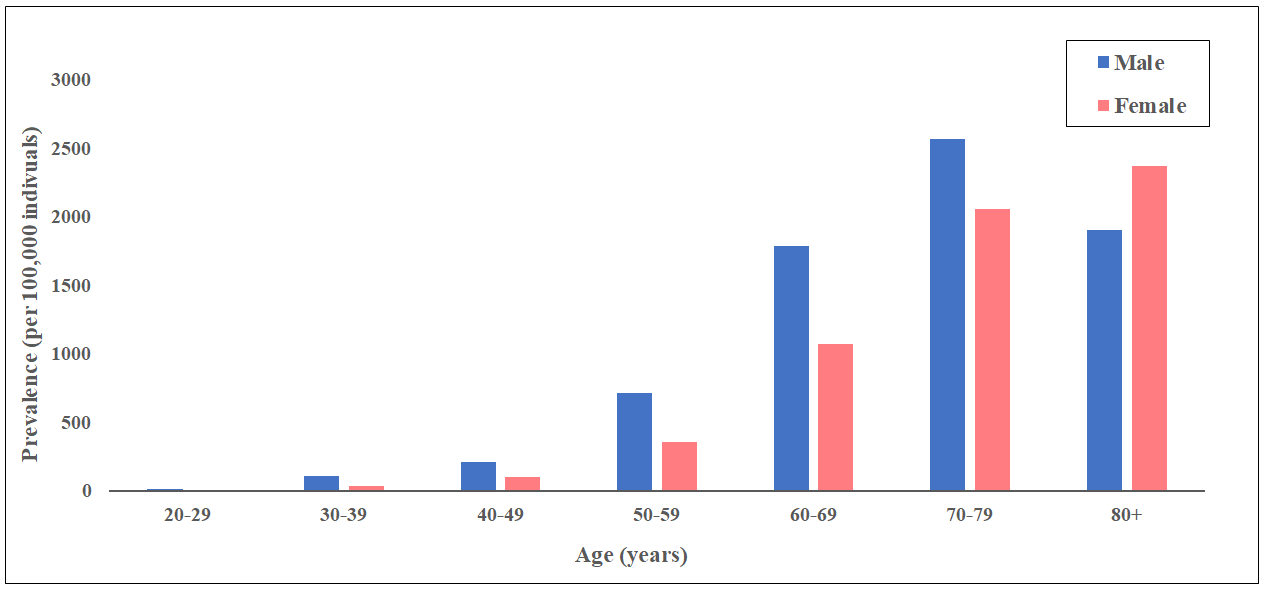
Prevalence of non-rheumatic calcific aortic valve disease in 2021.
Data obtained from the Global Burden of Disease Study 2021.
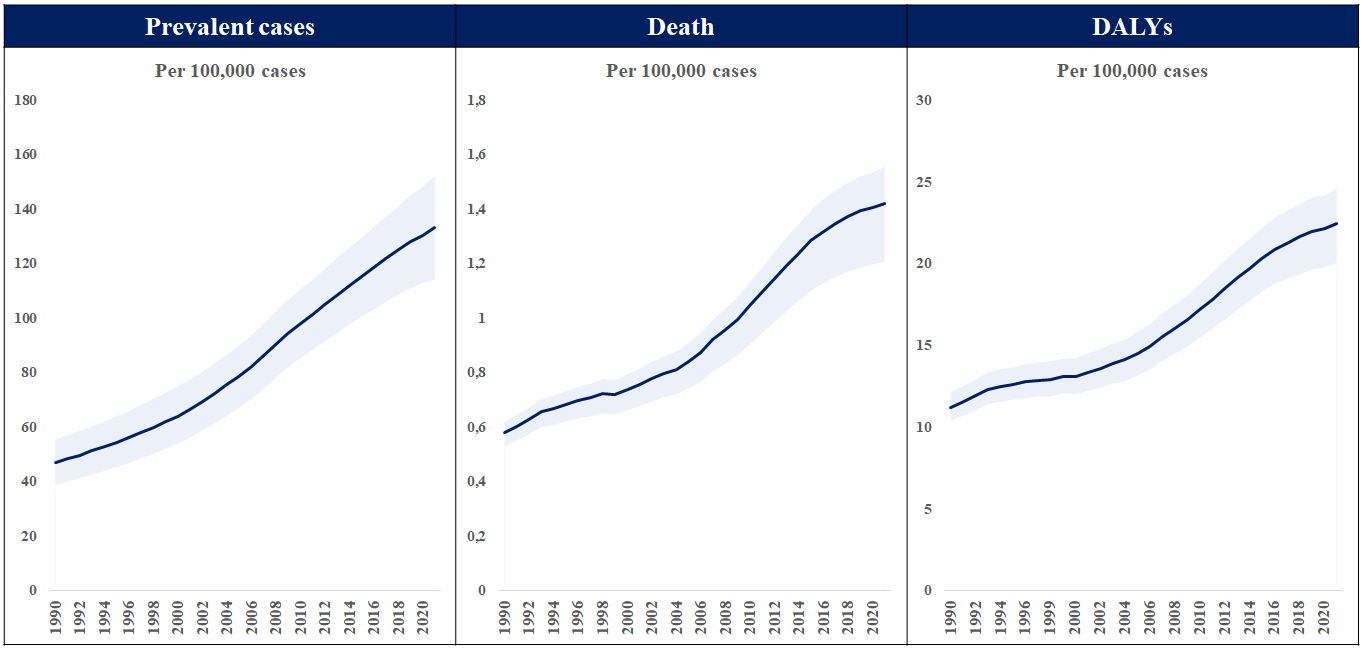
Total number of prevalent cases, death, and disability-adjusted life years (DALYs) to non-rheumatic calcific aortic valve disease between 1990 and 2021.
Data obtained from the Global Burden of Disease Study 2021.
The primary etiologies of AS comprise degenerative calcific stenosis (>80% in Europe/US), bicuspid/congenital anomalies (10% in Europe, US), and rheumatic heart disease (<5 in Europe, US). Epidemiological, histopathological, and imaging studies suggest an underlying pathological process with features of both atherosclerosis and elastocalcinosis, including progressive fibro-calcific remodeling and thickening of the aortic valve leaflets caused by genetic factors, lipoprotein deposition and oxidation, chronic inflammation, and osteoblastic transformation of cardiac valve interstitial cells, , . Common risk factors for AS include age, male sex, diabetes, hypercholesterolemia, arterial hypertension, obesity, and chronic kidney disease. Bicuspid aortic valve is the most prevalent congenital heart condition in adults. In the neonatal bicuspid aortic valve, the trilaminar structure is compromised with significant increase in collagen fibers and proteoglycans, as well as fragmentation of elastin fibers, which are associated with early progression of valve deterioration, . Rheumatic AS is characterized by non-calcific thickening of the leaflets and fusion of the commissures. Although there has been a significant reduction in the global burden of disease over the past decades, the health-related burden of rheumatic heart disease remains high particularly in middle-income and low-income countries. For the same haemodynamic severity of AS, women and men have different pathophysiology of AS: women have less aortic valve calcification and more valvular fibrosis with denser connective tissue than men. These differences may be related to a higher incidence of hypertension in women and poorly understood interactions with sex hormones, although this remains to be elucidated.
AS is a progressive disease characterized by the presence of aortic valve thickening and calcification, resulting in reduced leaflet motion, haemodynamic left ventricular outflow obstruction, and increased afterload, . Chronic pressure overload produces various anatomical and physiological changes including left ventricular hypertrophy, left atrial enlargement, and pulmonary arterial hypertension, leading to AS-related and heart failure symptoms, including exertional dyspnea, angina, and syncope. Current American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) guidelines for the management of valvular heart disease base the diagnosis and classification of AS on the integration of clinical symptoms and echocardiographic assessment (Table 2), . The definition of severe AS, the target of replacement therapy, comprises transaortic velocity ≥4 m/sec, mean transvalvular pressure gradient ≥40 mmHg, or aortic valve area ≤1 cm2 , . Recently, a staging classification has been proposed to characterize the extent of extra-aortic valve damage (Figure 6), demonstrating that advanced stages of cardiac damage are strongly associated with an increased risk of adverse events after aortic valve replacement (AVR), . This classification has been validated in various independent cohorts, , , and there is a growing interest in the grading scheme to improve patient management and therapeutic decision-making.
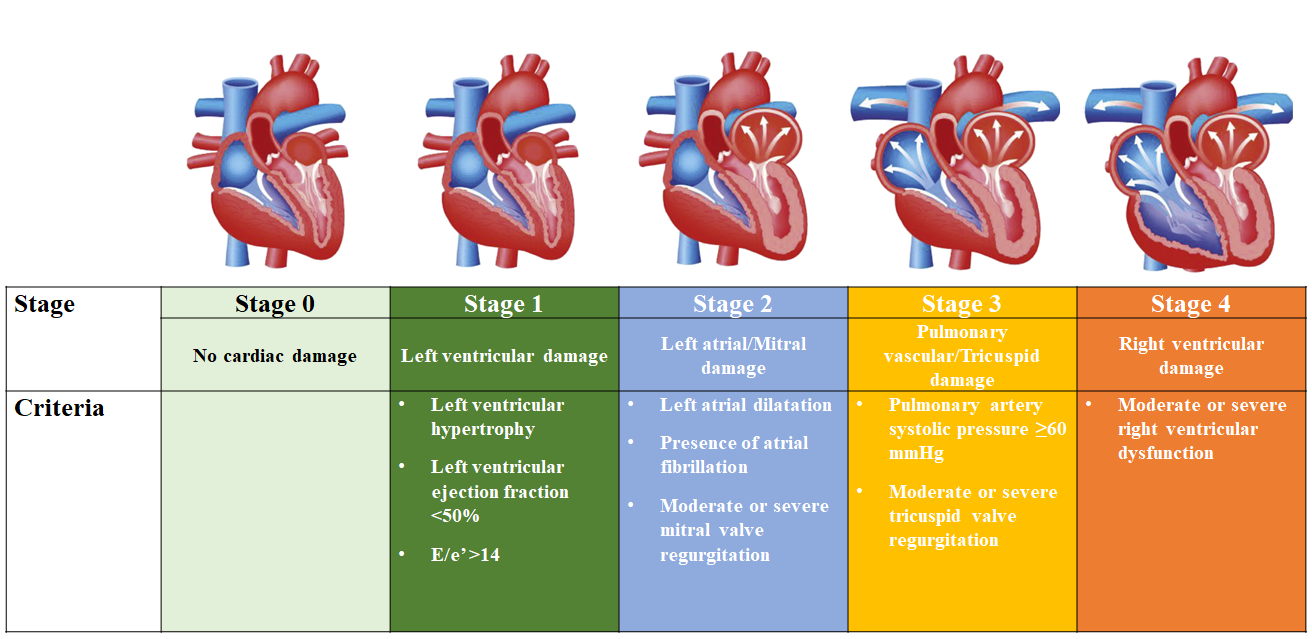
Proposed staging classification to quantify the extent of cardiac damage among patients with aortic stenosis.
Figures reproduced and modified from Généreux et al. J Am Coll Cardiol 2022;80:783-800.
Table 2. Classification of aortic stenosis
| 2020 ACC/AHA Valvular Heart Disease Guideline | Criteria | 2021 ESC/EACTS Valvular Heart Disease Guideline | |||
|---|---|---|---|---|---|
| Stage: Definition | Valve | Consequences | Stage | ||
| A: At risk of AS | Vmax < 2 m/s | None | Aortic valve sclerosis#1 | ||
| B: Progressive AS | Vmax 2.0-2.9 m/s or mPG <20 mmHg |
Early LV diastolic dysfunction Normal LVEF |
Mild AS#2 | ||
| Vmax 3.0-3.9 m/s or mPG 20-39 mmHg | Moderate AS#3 | ||||
| C: Asymptomatic severe AS | Vmax ≥4 m/s or mPG ≥40 mmHg AVA <1 cm2 (or AVAi <0.6 cm2/m2) |
LV diastolic dysfunction Mild LV Hypertrophy Normal LVEF |
Severe AS#4 | ||
| C1: Asymptomatic severe AS | |||||
| C2: Asymptomatic severe AS with LV systolic dysfunction | LVEF <50% | ||||
| D: Symptomatic severe AS | Vmax ≥4 m/s or mPG ≥40 mmHg AVA < 1 cm² (or AVAi < 0.6 cm²/m²) |
LV diastolic dysfunction LV hypertrophy Pulmonary hypertension may be present |
|||
| D1: Symptomatic severe high-gradient AS | |||||
| D2: Symptomatic severe low-flow, low-gradient with reduced LVEF | AVA <1 cm² with resting Vmax <4 m/s or mPG <40 mmHg Dobutamine stress echocardiography shows AVA <1 cm² with Vmax ≥4 m/s at any flow rate |
LV diastolic dysfunction Mild LV Hypertrophy LVEF ≥50% |
Low flow, low gradient AS with reduced ejection fraction#5 | ||
|
D3: Symptomatic severe low-gradient AS with normal LVEF or paradoxical low-flow severe AS |
AVA <1 cm2 with a Vmax <4 m/s or mPG <40 mmHg AND |
Increased LV relative wall thickness |
Low flow, low gradient AS with preserved ejection fraction | ||
|
#1 ESC/EACTS guidelines adopt criteria for Vmax <2.5 m/s. #2 ESC/EACTS guidelines adopt criteria for Vmax 2.6-2.9 m/s or mPG <20 mmHg or AVA > 1.5 cm2 or AVAi > 0.85 cm/m2 or Velocity ratio > 0.5. #3 ESC/EACTS guidelines adopt criteria for Vmax 3-4 m/s or mPG 20-40 mmHg or AVA 1-1.5 cm2 or AVAi 0.6-0.85 cm/m2 or Velocity ratio 0.25-0.5. #4 ESC/EACTS guidelines adopt criteria for Vmax ≥ 4 m/s or mPG ≥40 mmHg or AVA < 1 cm2 or AVAi < 0.6 cm/m2 or Velocity ratio < 0.25. #5 Additional criteria of SVI <35 ml/m2. ACC/AHA = American College of Cardiology/American Heart Association; AS = aortic stenosis; AVA = aortic valve area; AVAi = aortic valve area index to body surface area; ESC/EACTS = European Society of Cardiology and the European Association for Cardio-Thoracic Surgery; LV = left ventricular; LVEF = left ventricular ejection fraction; mPG = mean transvalvular gradient; SVI = stroke volume index to body surface area; Vmax = peak transvalvular velocity. |
|||||
Current ACC/AHA and ESC/EACTS guidelines for the management of valvular heart disease recommend aortic valve intervention (SAVR or TAVI) based on the severity of AS and associated symptoms (Table 3). Although there are differences, both guidelines are generally congruent regarding the timing of AVR, . The ACC/AHA guidelines recommend SAVR for patients aged <65 years or with life expectancy >20 years, TAVI for patients aged >80 years or with life expectancy <10 years, and shared decision-making for patients aged 65-80 years. The ESC/EACTS guidelines recommend SAVR in younger patients <75 years of age who are at low surgical risk (society of thoracic surgeons predicted risk of mortality [STS-PROM]/EuroSCORE II <4%) and TAVI in patients ≥75 years of age (Table 4 and Figure 7). Since the publication of these guidelines, several trials and updates comparing SAVR and TAVI have become available reporting on longer-term follow-up and new lower risk and younger populations which will impact future guideline recommendations, , , , , , , , , , , , .
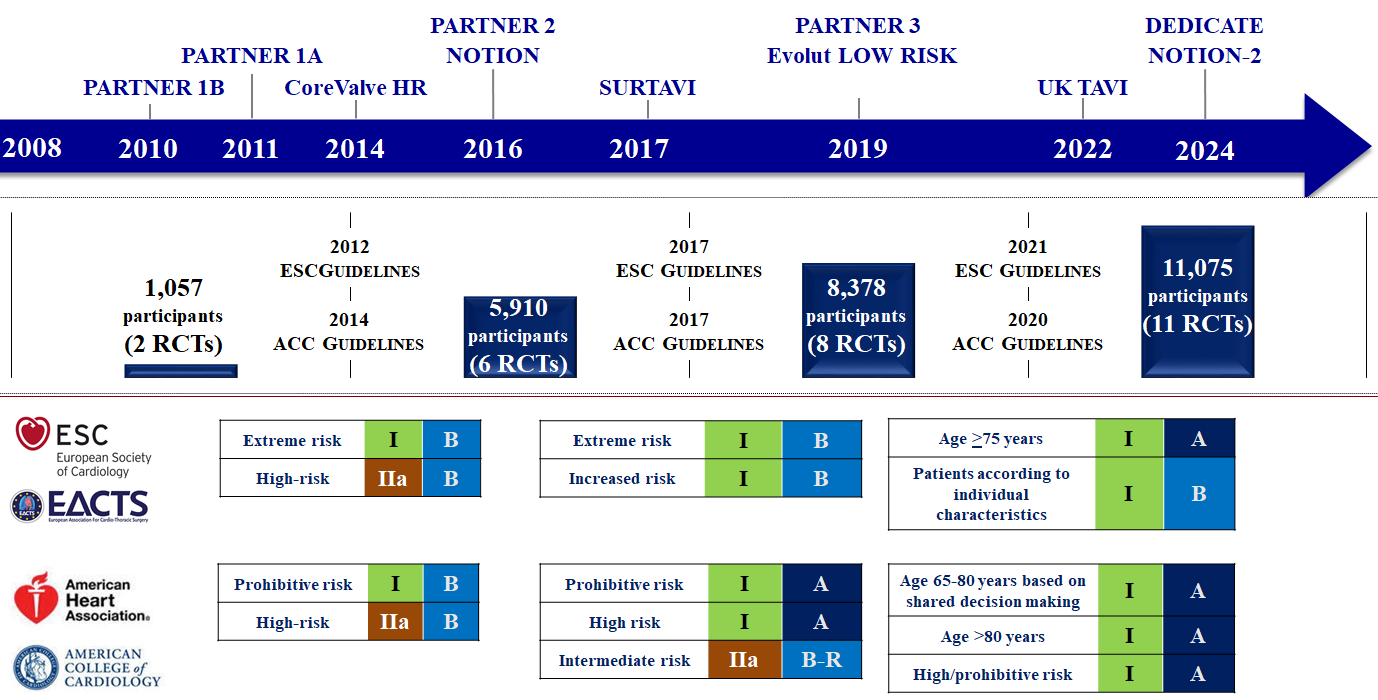
Evolution of transcatheter aortic valve implantation through evidence generation and guidelines.
Figures reproduced and modified from Windecker et al. Eur Heart J 2024;45(13):1104-1115.
Table 3. Guideline recommendations: Indications for aortic valve intervention
| Recommendations | 2020 AHA/ACC Valvular Heart Disease Guideline | 2021 ESC/EACTS Valvular Heart Disease Guideline | ||
|---|---|---|---|---|
| COR | LOE | COR | LOE | |
| Symptomatic severe AS | ||||
| Severe high-gradient AS | 1 | A | I | B |
| Classical low-flow low-gradient AS with evidence of flow (contractile) reserve | 1 | B-NR | I | B |
| Classical low-flow low-gradient AS without evidence of flow (contractile) reserve, particularly when CT calcium scoring confirms severe AS | 1 | B-NR | IIA | C |
| Paradoxical low-flow low-gradient AS*1 | 1 | B-NR | IIa | C |
| Asymptomatic severe AS | ||||
| LVEF <50% without another cause | 1 | B-NR | I | B |
| Symptoms on exercise test | 1 | A | I | C |
| A decrease in blood pressure on exercise test*2 | 2a | B-NR | IIa | C |
| LVEF <55% without another cause | / | IIa | B | |
| Very severe AS (Vmax >5.0 m/s or mean gradient ≥60 mmHg), low-surgical risk | 2a | B-R | IIa | B |
| Rapid progression (Vmax progression ≥0.3 m/s/year), low-surgical risk | 2a | B-NR | IIa | B |
| Markedly elevated BNP, low-surgical risk*3 | 2a | B-NR | IIa | B |
| Progressive decrease in LVEF on at least 3 serial imaging studies to <60% | 2b | B-NR | / | |
| Severe valve calcification (by CT)*4 | / | IIa | B | |
| Indications for other cardiac surgery | 1 | B-NR | I | C |
| Moderate AS | ||||
| Indications for other cardiac surgery | 2b | C-EO | IIa | C |
|
*1: AS is the most likely cause of symptoms in ACC/AHA guideline; after careful confirmation that the AS is severe in ESC/EACTS guideline. *2: A fall in systolic blood pressure of >10 mmHg from baseline during exercise testing in ACC/AHA and >20 mmHg in ESC/EACTS guidelines. *3: BNP >threefold age- and sex-corrected normal range confirmed by repeated measurements and without other explanation. *4: Severe AS very likely: (Agatston units): men ≥3000; women ≥1600; Severe AS likely: (Agatston units): men ≥2000; women ≥1200; Severe AS unlikely: (Agatston units): men <1600; women <800 ACC/AHA = American College of Cardiology/American Heart Association; AS = aortic stenosis; BNP = B-type natriuretic peptide; COR = class of recommendation; CT = computed tomography; ESC/EACTS = European Society of Cardiology and the European Association for Cardio-Thoracic Surgery; LOE = Level of evidence; LVEF = left ventricular ejection fraction; Vmax = peak transvalvular velocity |
||||
Table 4. Guideline recommendations: Mode of intervention
|
2020 AHA/ACC Valvular Heart Disease Guideline |
2021 ESC/EACTS Valvular Heart Disease Guideline |
||||
|---|---|---|---|---|---|
|
Recommendations |
COR |
LOE |
Recommendations |
COR |
LOE |
|
Transfemoral TAVI |
|||||
|
Patients who are >80 years of age or for younger patients with a life expectancy <10 years and no anatomical contraindication to transfemoral TAVI |
1 |
A |
Patients who are age ≥75 years of age, or at a high surgical risk (STS-PROME/EuroScore II >8%), or unsuitable for surgery |
I |
A |
|
Patients of any age with high or prohibitive surgical risk |
1 |
A |
|||
|
SAVR |
|||||
|
Patients who are <65 years of age or have a life expectancy >20 years |
1 |
A |
Patients who are <75 years of age and STS-PROME/EuroScore II <4%, operable and unsuitable for transfemoral TAVI |
I |
B |
|
Asymptomatic patients with severe AS and an abnormal exercise test, very severe AS, rapid progression, or an elevated BNP |
1 |
B-NR |
Patients undergoing other cardiac surgery |
I |
C |
|
Patients in whom a bioprosthetic valve is preferred but valve or vascular anatomy or other factors are not suitable for transfemoral TAVI |
1 |
A |
Patients with moderate AS undergoing other cardiac surgery (Heart team decision depending on patient-specific factors) |
IIa |
C |
|
Transfemoral TAVI or SAVR |
|||||
|
Patients who are 65-80 years of age and have no anatomic contraindication to transfemoral TAVI after shared decision making about the balance between expected patient longevity and valve durability |
1 |
A |
Remaining patients (Heart team decision depending on patient-specific factors) |
I |
B |
|
Asymptomatic patients with an LVEF <50% who are 65 to 80 years of age and have no contraindication to transfemoral TAVI |
1 |
B-NR |
|||
|
ACC/AHA = American College of Cardiology/American Heart Association; AS = aortic stenosis; BNP = B-type natriuretic peptide; COR = class of recommendation; ESC/EACTS = European Society of Cardiology and the European Association for Cardio-Thoracic Surgery; EuroScore = European System for Cardiac Operative Risk Evaluation; LOE = Level of evidence; LVEF = left ventricular ejection fraction; SAVR = surgical aortic valve replacement; STS-PROM = Society of Thoracic Surgeons Predicted Risk of Mortality; TAVI = transcatheter aortic valve implantation. |
|||||
Evidence generation has been established by comparison of TAVI, mostly with use of a balloon-expandable and a self-expanding THV device with conventional treatments in predominantly elderly patients with symptomatic severe AS across the entire risk spectrum (Figure 7). The cumulative evidence is based on 11 RCTs with more than 11,000 patients (Table 5, Table 6 and Table 7).
Table 5. Summary of randomized clinical trials of TAVI versus SAVR/medical treatment.
| Clinical Trial Author (year) |
Enrolment Year | Number of TAVI cohort (overall) |
Age (mean) | STS-PROM (mean) | TAVI Valve | TF access | Primary endpoint | Follow-up | TAVI | SAVR | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
PARTNER 1A |
2007-09 |
348 (699) |
83.6 ± 6.8 |
11.8 ± 3.3 |
SAPIEN THV |
70.1% |
All-cause death |
1-year |
All patients (N = 348) |
||
|
24.2% |
26.8% |
P = 0.44 |
|||||||||
|
Transfemoral cohort (N = 244) |
|||||||||||
|
22.2% |
26.4% |
P = 0.25 (P = 0.002)#1 |
|||||||||
|
Transapical cohort (N = 104) |
|||||||||||
|
29.0% |
27.9% |
P = 0.41 |
|||||||||
|
5-year |
All patients (N = 348) |
||||||||||
|
67.8% |
62.4% |
P = 0.76 |
|||||||||
|
Transfemoral cohort (N = 244) |
|||||||||||
|
63% |
64% |
P = 0.41 |
|||||||||
|
Transapical cohort (N = 104) |
|||||||||||
|
79% |
60% |
P = 0.067 |
|||||||||
|
PARTNER 1B#2 |
2007-09 |
179 (358) |
83.1 ± 8.6 |
11.2 ± 5.8 |
SAPIEN THV |
100% |
All-cause death |
1-year |
30.7% |
50.7% |
P <0.001 |
|
5-year |
71.8% |
93.6% |
P <0.0001 |
||||||||
|
CoreValve High risk |
2011-12 |
394 (797) |
83.2 ± 7.1 |
7.3 ± 3.0 |
CoreValve |
82.8% |
All-cause death |
1-year |
13.9% |
18.7% |
P = 0.04 (P<0.001)#1 |
|
3-year |
32.9% |
39.1% |
P = 0.068 |
||||||||
|
5-year |
55.3% |
55.4% |
P = 0.50 |
||||||||
|
PARTNER 2A |
2011-13 |
1011 (2032) |
81.5 ± 6.7 |
5.8 ± 2.1 |
SAPIEN XT |
76.7% |
Composite of all-cause death or disabling stroke |
2-year |
All patients (N = 1011) |
||
|
14.5% |
16.4% |
P = 0.25 (P = 0.001)#1 |
|||||||||
|
Transfemoral cohort (N = 775) |
|||||||||||
|
16.8% |
20.4% |
P = 0.05 |
|||||||||
|
Transthoracic cohort (N = 236) |
|||||||||||
|
27.7% |
23.4% |
P = 0.31 |
|||||||||
|
5-year |
All patients (N = 1011) |
||||||||||
|
47.9% |
43.4% |
P = 0.21 |
|||||||||
|
Transfemoral cohort (N = 775) |
|||||||||||
|
44.5% |
42.0% |
P = 0.80 |
|||||||||
|
Transthoracic cohort (N = 236) |
|||||||||||
|
59.3% |
48.3% |
P = 0.03 |
|||||||||
|
2 to 5 years |
Landmark analysis (N = 1011) |
||||||||||
|
36.3% |
29.5% |
(1.27 [1.06, 1.53])#3 |
|||||||||
|
SURTAVI |
2012-16 |
864 (1660) |
79.9 ± 6.2 |
4.4 ± 1.5 |
CoreValve, Evolut R |
100% |
All-cause death or disabling stroke |
2-year |
12.6% |
14.0% |
(95% credible interval [Bayesian analysis] for difference -5.2, 2.3]) posterior probability of non-inferiority >0.999 |
|
5-year |
31.3% |
30.8% |
P = 0.85 |
||||||||
|
NOTION |
2009-13 |
145 (280) |
79.2 ± 4.9 |
2.9 ± 1.6 |
CoreValve |
96.5% |
All-cause death, stroke, myocardial infarction |
1-year |
13.1% |
16.3% |
P = 0.43 |
|
5-year |
38.0% |
36.3% |
P = 0.86 |
||||||||
|
8-year |
54.5% |
54.8% |
P = 0.94 |
||||||||
|
10-year |
65.5% |
65.5% |
P = 0.9 |
||||||||
|
UK-TAVI Trial |
2014-2018 |
458 (913) |
81 (79-84) |
2.6 (2.0-3.5) |
Any CE-mark devices |
92.0% |
All-cause death |
1-year |
4.6% |
6.6% |
P <0.001#1 |
|
DEDICATE |
2017-2022 |
701 (1414) |
74.3 ± 4.6 |
1.8 (1.2-2.4) |
Any CE-mark devices |
97.3% |
All-cause death, stroke |
1-year |
5.4% |
10% |
P <0.001#1 |
|
PARTNER 3 |
2016-17 |
496 (1000) |
73.3 ± 5.8 |
1.9 ± 0.6 |
SAPIEN 3 |
100% |
All-cause death, stroke, rehospitalization |
1-year |
8.5% |
15.1% |
P<0.001 (P = 0.001)#1 |
|
2-year |
11.5% |
17.4% |
P = 0.007 |
||||||||
|
5-year |
22.8% |
27.2% |
P = 0.07 |
||||||||
|
Evolut low risk |
2016-18 |
734 (1468) |
74.0 ± 5.9 |
1.9 ± 0.7 |
CoreValve, Evolut R/PRO |
99.6% |
All-cause death, disabling stroke |
2-year |
5.0% |
6.6% |
(-1.5[-4.9, 1.8])#4 posterior probability of non-inferiority >0.999 |
|
3-year |
7.4% |
10.4% |
P = 0.051 |
||||||||
|
4-year |
10.7% |
14.1% |
P = 0.05 |
||||||||
|
5-year |
15.5% |
16.4% |
P = 0.47 |
||||||||
|
NOTION-2 |
2016-2023 |
187 (370) |
71.1 ± 3.1 |
1.1 (0.9-1.5) |
Any CE-mark devices |
100% |
All-cause mortality, stroke, rehospitalization |
1-year |
All patients (N = 187) |
||
|
10.2% |
7.1% |
P = 0.3 |
|||||||||
|
Tricuspid cohort (N = 138) |
|||||||||||
|
8.7% |
8.3% |
P = 0.9 |
|||||||||
|
Bicuspid cohort (N = 49) |
|||||||||||
|
14.3% |
3.9% |
P = 0.07 |
|||||||||
|
The results provided are from the intention-to-treat analysis except for NOTION trial at 1 year with as-treated analysis. Numbers, mean age, and mean STS score are of TAVI cohorts. Blue indicates results with non-inferiority of TAVI versus SAVR or no significant difference between TAVI and SAVR. Yellow indicates results with statistically better outcomes of TAVI over SAVR or superiority of TAVI over SAVR. Red indicates results with statistically better outcomes of SAVR over TAVI. #1: P value for non-inferiority test. #2: Results are provided with differences between TAVI and standard treatment. #3: Results are provided with hazard ratio and 95% confidence intervals (CI). #4: Results are provided with differences (TAVI-SAVR) and 95% Bayesian credible interval (BCI). STS-PROM = Society of Thoracic Surgeons Predicted Risk of Mortality; TA=transapical; TAVI = transcatheter aortic valve implantation; SAVR = surgical aortic valve replacement. |
|||||||||||
Table 6. Short-term echocardiographic and clinical outcomes comparing TAVI and SAVR in randomized clinical trials.
|
Trial |
Echocardiographic outcomes |
30-day Clinical outcomes |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
AVA |
mPG |
PVR |
All-cause death |
Major stroke |
Major bleeding |
Permanent pacemaker |
||||||||||
|
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
|||
|
PARTNER 1A |
1.7 ± 0.5 |
1.5 ± 0.4 |
9.9 ± 4.8 |
10.8 ± 5.0 |
12.2% |
0.9% |
3.4% |
6.5% |
11.0% |
3.2% |
9.3% |
19.5% |
3.8% |
3.6% |
||
|
P = 0.001 |
P = 0.004 |
P<0.001 |
P = 0.07 |
P = 0.20 |
P<0.001 |
P = 0.89 |
||||||||||
|
PARTNER 1B#1
|
1.5 ± 0.4 |
0.8 ± 0.2 |
11.4 ± 7.0 |
33.1 ± 12.6 |
12% |
0% |
5.0% |
2.8% |
5.0% |
1.1% |
16.8% |
3.9% |
3.4% |
5.0% |
||
|
NA |
NA |
NA |
P = 0.41 |
P = 0.06 |
P<0.001 |
P = 0.60 |
||||||||||
|
CoreValve High risk |
1.95 ± 0.56 |
1.60 ± 0.51 |
8.88 ± 3.87 |
11.71 ± 5.71 |
9.0% |
1.0% |
3.3% |
4.5% |
3.9% |
3.1% |
28.1% |
34.5% |
19.8% |
7.1% |
||
|
P<0.001 |
P<0.001 |
P<0.001 |
P = 0.43 |
P = 0.55 |
P = 0.05 |
P<0.001 |
||||||||||
|
PARTNER 2A
|
1.7 ± 0.5 |
1.5 ± 0.4 |
9.7 ± 3.5 |
10.9 ± 4.3 |
3.7% |
0.6% |
3.9% |
4.1% |
3.2% |
4.3% |
10.4% |
43.4% |
8.5% |
6.9% |
||
|
<0.001 |
<0.001 |
P<0.001 |
P = 0.78 |
P = 0.20 |
P<0.001 |
P = 0.17 |
||||||||||
|
SURTAVI#2 |
2.1 ± 0.6 |
1.8 ± 0.6 |
8. 9± 4.1 |
12.4 ± 5.7 |
3.4% |
0.3% |
2.2% |
1.7% |
1.2% |
2.5% |
12.1% |
9.3% |
25.9% |
6.6% |
||
|
NA |
NA |
NA |
95%CI |
95%CI |
95%CI |
95%CI |
||||||||||
|
NOTION#3
|
1.7 |
1.4 |
8.3 |
12.2 |
15.7% |
0.9% |
2.1% |
3.7% |
1.4%+ |
3.0%+ |
11.3% |
20.9% |
34.1% |
1.6% |
||
|
P<0.001 |
P<0.001 |
P<0.001 |
P = 0.43 |
P = 0.37 |
P = 0.03 |
P<0.001 |
||||||||||
|
UK-TAVI Trial |
1.53 ± 0.48 |
1.51 ± 0.48 |
10.36 ± 4.82 |
10.01 ± 5.12 |
2.4% |
0.9% |
1.8% |
0.9% |
2.4% |
2.3% |
5.5% |
19.5% |
11.0% |
6.7% |
||
|
0.04 |
0.31 |
5.37 |
1.91 |
1.05 |
0.27 |
1.72 |
||||||||||
|
DEDICATE#5 |
1.8 (1.5-2.1) |
1.7 (1.4-2.0) |
11.0 (8.0-14.9) |
11.0 (8.0-14.2) |
1.7% |
0.7% |
0.7% |
1.5% |
0.6% |
1.7% |
3.5% |
12.7% |
8.3% |
4.1% |
||
|
-0.1 |
0.0 |
NA |
0.55 |
0.35 |
0.26 |
2.09 |
||||||||||
|
PARTNER 3#5 |
1.7 |
1.8 |
12.8 |
11.2 |
0.8% |
0% |
0.4% |
1.1% |
0% |
0.4% |
3.6% |
24.5% |
6.5% |
4.0% |
||
|
NA |
NA |
NA |
0.37 |
0.00 |
0.12 |
1.66 |
||||||||||
|
Evolut low risk#2
|
2.2 ± 0.6 |
2.0 ± 0.6 |
8.4 ± 3.5 |
10.5 ± 4.0 |
3.4% |
0.4% |
0.5% |
1.3% |
0.5% |
1.7% |
2.4% |
7.5% |
17.4% |
6.1% |
||
|
NA |
NA |
NA |
-0.8 |
-1.2 |
-5.1 |
11.3 |
||||||||||
|
NOTION-2#5 |
1.8 |
1.6 |
10.6 |
12.6 |
4.7% |
0% |
0.5% |
1.1% |
0.5% |
1.1% |
3.7% |
16.9% |
12.8% |
4.6% |
||
|
NA |
NA |
P = 0.005 |
0.5 |
0.5 |
0.2 |
2.9 |
||||||||||
|
The results of PARTNER 1A, PARTNER 2A, PARTNER 2B, PARTNER 3, SURTAVI, UK-TAVI Trial, DEDICATE, PARTNER 3, and NOTION-2 are provided from intention-to-treat analyses. The results of U.S. CoreValve High risk, NOTION, and Evolut low risk are provided from as-treated analyses. Blue indicates results with no significant difference between TAVI and SAVR. Yellow indicates results with statistically better outcomes of TAVI over SAVR. Red indicates results with statistically better outcomes of SAVR over TAVI. #1: Results are provided with differences between TAVI and standard treatment. #2: Results are provided with differences (TAVI-SAVR) and 95% Bayesian credible interval (BCI). #3: Echocardiographic data at discharge or 30 days was not provided. The echocardiographic results are at 3-month follow-up. #4: Echocardiographic data at discharge or 30 days was not provided. The echocardiographic results are at 6-week follow-up. #5: Results are provided with hazard ratios and 95% confidence intervals (CI). a: Treatment effect and 95% confidence intervals (CI) (TAVI vs. SAVR). b: median difference and 95% confidence intervals (CI) (TAVI vs. SAVR). + : Any stroke. AVA = aortic valve area; mPG = mean transvalvular gradient; PVR = paravalvular regurgitation; SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation. |
||||||||||||||||
Table 7. Long-term clinical outcomes comparing TAVI and SAVR in randomized clinical trials.
|
Period |
1-year |
Longest follow-up |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Clinical outcome |
All-cause death |
Cardiovascular death |
Rehospitalization |
Major stroke |
Reintervention |
All-cause death |
Cardiovascular death |
Rehospitalization |
Major stroke |
Reintervention |
||||||||||||
|
TAVI vs. SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
TAVI |
SAVR |
||
|
PARTNER 1A |
24.2% |
26.8% |
14.3% |
13.0% |
18.2% |
15.5% |
5.1% |
2.4% |
/ |
67.8% |
62.4% |
53.1% |
47.6% |
42.3% |
34.2% |
10.4% |
11.3% |
/ |
||||
|
P = 0.44 |
P = 0.63 |
P = 0.38 |
P = 0.07 |
P = 0.76 |
P = 0.67 |
P = 0.17 |
P = 0.61 |
|||||||||||||||
|
PARTNER 1B#1 |
30.7% |
49.7% |
19.6% |
41.9% |
22.3% |
44.1% |
7.8% |
3.9% |
/ |
71.8% |
93.6% |
71.8% |
92.7% |
47.6% |
87.3% |
16.0%+ |
18.2%+ |
/ |
||||
|
P<0.001 |
P<0.001 |
P<0.0001 |
P = 0.18 |
P<0.0001 |
P<0.0001 |
P<0.0001 |
P = 0.555 |
|||||||||||||||
|
CoreValve High risk
|
14.2% |
19.1% |
10.4% |
12.8% |
16.5% |
13.9% |
5.8% |
7.0% |
1.9% |
0.0% |
55.3% |
55.4% |
39.7% |
39.5% |
37.5% |
31.5% |
12.3% |
13.2% |
3.0% |
1.1% |
||
|
P = 0.04 |
P = 0.31 |
NA |
P = 0.59 |
P = 0.01 |
P = 0.50 |
P = 0.80 |
P = 0.08 |
P = 0.49 |
P = 0.04 |
|||||||||||||
|
PARTNER 2A
|
12.3% |
12.9% |
7.1% |
8.1% |
14.8% |
14.7% |
5.0% |
5.8% |
1.2% |
0.5% |
46.0% |
42.1% |
29.4% |
27.8% |
33.3% |
25.2% |
9.8% |
8.6% |
3.2% |
0.8% |
||
|
P = 0.69 |
P = 0.40 |
P = 0.92 |
P = 0.46 |
P = 0.10 |
HR 1.09 [0.95-1.25] |
HR 1.02 [0.85-1.23] |
HR 1.28 [1.07-1.53] |
HR 1.05 [0.77-1.44] |
HR 3.28 [1.32-8.13] |
|||||||||||||
|
SURTAVI |
7.0% |
6.8% |
4.8% |
5.5% |
9.0% |
8.7% |
2.2% |
3.7% |
2.0% |
0.5% |
30.0% |
28.7% |
17.8% |
17.4% |
23.9% |
20.8% |
4.1% |
5.8% |
3.5% |
1.9% |
||
|
95% credible interval for the difference -2.3, 2.7 |
95% credible interval for the difference -2.9, 1.5 |
95% credible interval for the difference -2.6, 3.1 |
95% credible interval for the difference -3.2, 0.2 |
95% credible interval for the difference 0.3, 2.6 |
P = 0.55 |
P = 0.84 |
P = 0.13 |
P = 0.11 |
P = 0.02 |
|||||||||||||
|
NOTION |
4.9% |
7.5% |
4.3% |
7.5% |
/ |
2.9%+ |
4.6%+ |
/ |
62.7% |
64.0% |
49.5% |
51.2% |
/ |
9.7%+ |
16.4%+ |
4.3% |
2.2% |
|||||
|
P = 0.38 |
P = 0.25 |
P = 0.44 |
P = 0.8 |
P = 0.7 |
P = 0.1 |
P = 0.3 |
||||||||||||||||
|
UK-TAVI Trial |
4.6% |
6.6% |
2.8% |
3.3% |
/ |
5.2%+ |
2.6%+ |
2.2% |
1.1% |
/ |
||||||||||||
|
Adjusted HR 0.69 [0.38-1.26] |
Adjusted HR 0.86 [0.40-1.83] |
Adjusted HR 1.98 [0.95-4.11] |
Adjusted HR 1.98 [0.72-5.42] |
|||||||||||||||||||
|
DEDICATE |
2.6% |
6.2% |
2.0% |
4.4% |
12.2% |
13.3% |
1.3% |
3.1% |
0.6% |
0.3% |
/ |
|||||||||||
|
HR 0.43 (0.24-0.73) |
HR 0.47 (0.24-0.86) |
HR 0.89 (0.66-1.20) |
HR 0.42 (0.19-0.88) |
HR 1.70 (0.38-9.78) |
||||||||||||||||||
|
PARTNER 3 |
1.0% |
2.5% |
0.8% |
2.0% |
7.3% |
11.0% |
0.2% |
0.9% |
0.6% |
0.5% |
10.0% |
8.2% |
5.5% |
5.1% |
13.7% |
17.4% |
2.9% |
2.7% |
2.6% |
3.0% |
||
|
HR 0.41 (0.14-1.17) |
HR 0.40 (0.12-1.30) |
HR 0.65 (0.42-1.0) |
HR 0.22 (0.03-2.0) |
HR 1.33 (0.22-7.95) |
HR 1.23 (0.79-1.90) |
HR 1.08 (0.61-1.92) |
HR 0.75 (0.54-1.05) |
HR 1.03 (0.46-2.30) |
HR 0.86 (0.39-1.92) |
|||||||||||||
|
Evolut low risk |
2.4% |
3.0% |
1.7% |
2.6% |
3.2% |
6.5% |
0.8% |
2.4% |
0.7% |
0.6% |
13.5% |
14.9% |
7.2% |
9.3% |
13.9% |
15.1% |
3.6% |
4.0% |
3.3% |
2.5% |
||
|
Difference -0.6 95% BCI [-2.6, 1.3] |
Difference -0.9 95% BCI [-2.7, 0.7] |
Difference -3.4 95% BCI [-5.9, -1.0] |
Difference -1.6 95% BCI [-3.1, -0.3] |
Difference 0.0 95% BCI [-1.0, 0.9] |
P = HR 0.88 (0.66-1.17) |
P = HR 0.75 (0.51-1.11) |
P = HR 0.89 (0.67-1.19) |
P = HR 0.85 (0.49-1.49) |
HR 1.30 (0.66-2.56) |
|||||||||||||
|
NOTION-2 |
2.1% |
1.1% |
2.1% |
1.1% |
3.7% |
4.9% |
1.6% |
1.1% |
1.1% |
2.2% |
/ |
|||||||||||
|
HR 2.0 |
HR 2.0 |
HR 0.7 |
HR 1.5 |
HR 0.5 |
||||||||||||||||||
|
Only 1-year results are shown in the UK-TAVI, DEDICATE, and NOTION-2 trials. 1- and 5-year results are shown in the PARTNER 1A and 1B, PARTNER 2, PARTNER 3, CoreValve High Risk, SURTAVI, and Evolut low risk trials. 1- and 10-year results are shown in the NOTION trial. Results of the CoreValve High risk, NOTION, and Evolut low risk trials are provided from as-treated analyses. Blue indicates results with no significant difference between TAVI and SAVR. Yellow indicates results with statistically better outcomes of TAVI over SAVR. Red indicates results with statistically better outcomes of SAVR over TAVI. #1: Results are provided with differences between TAVI and standard treatment. + : Any stroke. BCI = Bayesian credible interval; HR = hazard ratio; SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation. |
||||||||||||||||||||||
The PARTNER 1 trial consisted of 2 randomized cohorts: Cohort A, which compared transfemoral/transapical TAVI with the Edwards SAPIEN balloon-expandable valve system (N = 348) or SAVR (N = 351) in high-risk patients; and Cohort B, which randomized inoperable patients to either conservative treatment – including balloon aortic valvuloplasty (N = 179) or transfemoral TAVI (N = 179). The primary endpoint was all-cause death at 1 year.
In Cohort B (mean age: 83 years, STS-PROM: 11.2%), TAVI was associated with a 20% absolute risk reduction in all-cause mortality compared with conservative treatment at 1 year (30.7% vs. 50.7%, hazard ration [HR] 0.55, 95% confidence interval [CI] 0.40-0.74, P <0.001) (NNT=5). The follow-up data reported up to 5 years showed TAVI to maintain superiority over medical treatment. At the end of 5-year follow-up, overall mortality was 71.8% in the TAVI group compared with 93.8% in the medical treatment group (HR 0.50, 95% CI 0.39-0.65, P <0.001).
In cohort A (mean age: 84 years, STS-PROM: 11.8%), TAVI was non-inferior to SAVR in terms of all-cause death at 1 year (24.2 vs. 26.8%, Pnon-inferiority = 0.001). At 5-year follow-up, the risk of all-cause death remained similar between TAVI and SAVR (67.8% vs. 62.4%, HR 1.04, 95% CI 0.86-1.24). In a stratified analysis by access site, there was no difference in mortality between transfemoral TAVI and SAVR (63% vs. 64%, P = 0.41), while transapical TAVI was associated with a numerically higher risk of mortality (79% vs. 60%, P = 0.067) compared with SAVR at 5 years. The risk of repeat hospitalization (42.3% vs. 34.2%, P = 0.17), stroke (10.4% vs. 11.3%, P = 0.61), and myocardial infarction (2.9% vs. 5.9%, P = 0.15), and functional status (New York Heart Association [NYHA] class I or II: 85% vs. 81%, P = 0.57) were comparable between the groups up to 5 years. Major vascular complications were more frequent after TAVI (11.9% vs. 4.7%, P = 0.0002), while major bleeding more frequently occurred after SAVR (26.6% vs. 34.4%, P = 0.003).
The CoreValve US High Risk trial was the first trial comparing SAVR with TAVI using the self-expanding CoreValve system in 795 high-risk patients (mean age: 83 years, STS-PROM: 7.3%). The primary endpoint was all-cause death at 1 year. TAVI was predominantly performed via transfemoral access (82.8%). TAVI was associated with a significantly lower risk of all-cause death at 1 year than SAVR (14.2% vs. 19.1%, Pnon-inferiority <0.001, Psuperiority = 0.04). At 5 years, both TAVI and SAVR resulted in similar survival outcomes (all-cause death: 55.3% vs. 55.4%, HR 0.93, 95% CI 0.77 to 1.14, P = 0.5). The risk of repeat hospitalization (37.5% vs. 31.5%, P = 0.08), major stroke (12.3% vs. 13.2%, P = 0.49), and myocardial infarction (3.1% vs. 3.3%, P = 0.93), and functional status (NYHA class mean of 1.3 in both groups) were comparable between the groups up to 5 years, while major vascular complications (7.1% vs. 2.0%, P = 0.001) and repeat aortic valve intervention (3.0% vs. 1.1%, P = 0.04) occurred more frequently in the TAVI group and major bleedings was more frequently observed (35.9% vs. 43.3%, P = 0.05) in the SAVR group throughout 5-year follow-up.
The PARTNER 2 trial randomly assigned 2,032 intermediate-risk patients (mean age: 82 years, STS-PROM: 5.8%) to SAVR or TAVI with the balloon-expandable SAPIEN XT system. Patients were stratified in cohorts according to access route (transfemoral 76.3% or transthoracic 23.7%). The primary endpoint was the composite of all-cause death or disabling stroke and TAVI was non-inferior to SAVR in terms of the primary endpoint at 2 years (19.3% vs. 21.1%, Pnon-inferiority = 0.001). In the transfemoral access cohort, TAVI resulted in a lower rate of death or disabling stroke than SAVR (HR 0.79, 95% CI 0.62-1.00, P = 0.05), while in the transthoracic access cohort, outcomes were similar between the two groups (HR 1.21, 95% CI 0.84-1.74, P = 0.31). At 5-year follow-up, no significant difference was observed in the primary endpoint between the TAVI group and the SAVR group (47.9% vs. 43.4%, HR 1.09. 95% CI 0.95-1.25, P = 0.21). Results were similar for the transfemoral access cohort (44.5% vs. 42.0%, HR 1.02, 95% CI 0.87-1.20), while TAVI was associated with an increased risk of the primary endpoint compared to SAVR in the transthoracic-access cohort (59.3% vs. 48.3%, HR 1.32, 95% CI 1.02-1.71). Improvements in health status (NYHA class I or II: 89.0% vs. 92.7%; average increase in the Kansas City Cardiomyopathy Questionnaire [KCCQ] Overall Summary score: 19.6 points and 20.5 points) were also similar at 5 years. More patients in the TAVI group than in the SAVR group had aortic-valve reintervention (3.2% vs. 0.8%, HR 3.28, 95% CI 1.32-8.13) and repeat hospitalization (33.3% vs. 25.2%, HR 1.28, 95% CI 1.07-1.53) at 5 years.
The SURTAVI trial (N = 1,746) was a randomized trial designed to compare the safety and efficacy of TAVI with the CoreValve (84%) or the Evolut R (16%) self-expanding valve system and SAVR in intermediate-risk patients (mean age: 80 years, STS-PROM: 4.4%). TAVI was predominantly performed via transfemoral access (94%), while subclavian (2%) or direct aortic (4%) approaches were used in patients with unsuitable iliofemoral anatomy. The primary endpoint was the composite of all-cause death or disabling stroke at 2 years. Consistent with prior studies, TAVI was non-inferior to SAVR for the primary endpoint (12.6% vs. 14.0%, difference -1.4 percentage points, 95% Bayesian credible interval for difference [BCI] -5.2 to 2.3%, posterior probability of non-inferiority >0.999). At 5 years, rates of all-cause death (30.0% vs. 28.7%, P = 0.55), rehospitalization (23.9% vs. 20.8%, P = 0.13), and major stroke (4.1% vs. 5.8%, P = 0.11) were similar between the TAVI and SAVR groups, while repeat aortic valve intervention occurred more frequently in the TAVI group (3.5%vs. 1.9%, P = 0.02).
The NOTION I trial randomized patients with severe AS (age ≥70 years and no evidence of significant coronary artery disease) irrespective of surgical risk to TAVI with the self-expanding CoreValve valve system or SAVR. A total of 280 patients (mean age 79 years, STS-PROM: 2.9%) were included, and the majority of patients (81.8%) were considered low-risk patients. The primary endpoint was the composite of all-cause death, stroke, or myocardial infarction at 1 year. There were no differences between TAVI and SAVR for the primary endpoint (13.1% vs. 16.3%, Psuperiority = 0.43) or any of its components at 1 year. At 10 years of follow-up, the risk of the composite primary outcome of all-cause mortality, stroke, or myocardial infarction was 65.5% in the TAVI group and 65.5% in the SAVR group with no significant difference (HR = 1.0, 95% CI 0.7-1.3, P = 0.9). Although there was no difference in the rate of the components of the primary endpoint, TAVI was associated with a higher rate of new permanent pacemaker implantation (44.7% vs. 14.0, P <0.01) and SAVR had higher rate of new-onset atrial fibrillation (52.0% vs. 74.1%, P <0.01). The rate of aortic valve reintervention was low and similar for the two types of AVR (TAVI 4.3% and SAVR 2.2%, P = 0.3).
The UK-TAVI trial was an investigator-initiated, multicenter, RCT involving all National Health Service hospitals performing TAVI in the UK, including 913 patients aged 70 years or older with severe, symptomatic AS and moderately increased operative risk due to age or comorbidity (median age: 81, STS-PROM: 2.6%). TAVI was performed using any valve with a CE mark (balloon expandable valves 57.3%, self-expanding valves: 31.6%, mechanically-expanding valve: 9.6%) and via any access route (transfemoral access: 92.0%). At 1 year, the primary endpoint of all-cause mortality was 4.6% in the TAVI group and 6.6% in the SAVR group (Pnon-inferiority <0.001). TAVI was associated with a reduced risk of major bleeding (7.2% vs. 20.2%), whereas vascular complications (10.3% vs. 2.4%), conduction disturbances requiring pacemaker implantation (14.2% vs. 7.3%), and mild or moderate aortic regurgitation (2.3% vs. 0.6%) were more frequently observed in the TAVI group.
The PARTNER 3 trial was a randomized trial (N = 1,000) that compared transfemoral TAVI using the balloon-expandable SAPIEN 3 valve system with SAVR in low-risk patients (mean age: 73 years, STS-PROM: 1.9%). The primary endpoint was the composite of all-cause death, any stroke, or repeat hospitalization at 1 year. The rate of the primary endpoint was lower in the TAVI group than in the SAVR group (8.5% vs. 15.1%, absolute difference -6.6 percentage points, 95%CI -10.8 to -2.5, Pnon-inferiority <0.001, HR 0.54, 95% CI 0.37 to 0.79, Psuperiority = 0.001). TAVR resulted in a lower rate of stroke (0% vs. 0.4%, P <0.001) and new-onset atrial fibrillation (5.0% vs. 39.5%, P <0.001) at 30 days, while there were no significant between-group differences in major vascular complications, new permanent pacemaker insertions, or moderate or severe paravalvular regurgitation (PVR). At 5-year follow-up, rates of the composite clinical outcome (22.8% vs. 27.2%, HR 0.79, 95% CI 0.61-1.02) and its components (all-cause death: 10.0% vs. 8.2%, HR 1.23, 95% CI 0.79-1.90; any stroke: 5.8% vs. 6.4%, HR 0.87, 95% CI 0.51-1.48, rehospitalization: 13.7% vs. 17.4%, HR 0.75, 95% CI 0.54-1.05) were similar among patients assigned to TAVI and SAVR, respectively. The rate of aortic valve reintervention (2.6% in the TAVI group and 3.0% in the SAVR group) and the proportion of patients who were alive with a KCCQ Overall Summary score of 75 or higher (indicative of being well) were similar in the two groups (71.0% in the TAVI group and 71.9% in the SAVR group), while valve thrombosis according to the Valve Academic Research Consortium (VARC)-3 definition was higher in the TAVR group (2.5% vs. 0.2%, HR 10.52, 95% CI 1.37-80.93). This trial is planned to be followed for 10 years.
The Evolut Low Risk trial was a randomized trial (N = 1,468) that compared TAVI with the self-expanding CoreValve (3.6%), Evolut R (74.1%), or Evolut PRO (22.3%) system with SAVR in low-risk patients (mean age: 73 years, STS-PROM: 1.9%). The primary endpoint was the composite of all-cause death or disabling stroke at 2 years. TAVI was performed predominantly using transfemoral access (99.0%). When 850 patients had reached 1-year follow-up, data were analysed using Bayesian methods. At 2 years, TAVI met non-inferiority compared to SAVR in terms of the primary endpoint (5.3% vs. 6.7%, difference -1.4 percentage points, 95% BCI -4.0 to 2.1, posterior probability of non-inferiority >0.999). TAVI resulted in a lower incidence of disabling stroke (0.5% vs. 1.7%, difference -1.2, 95% BCI -2.4 to -0.2), bleeding complications (7.7% vs. 35.4%. difference -5.1, 95% BCI -7.5 to -2.9), acute kidney injury (0.9% vs. 2.8%, difference -1.8, 95% BCI -3.4 to -0.5), and new-onset atrial fibrillation (7.7% vs. 35.4%, difference -27.7, 95% BCI -31.8 to -23.6) at 30 days, as compared to SAVR. Conversely, the rates of new permanent pacemaker implantation (17.4% vs. 6.1%, difference 11.3, 95% BCI 8.0 to 14.7) were higher in the TAVI group than in the SAVR group. At 5 years, there was no significant difference in the primary endpoint (15.5% vs. 16.4%; HR: 0.90; 95% CI: 0.69-1.18; P = 0.47) and its components (all-cause death: 13.5% vs. 14.9%; HR: 0.88; 95% CI: 0.66-1.17; P = 0.39; disabling stroke: 3.6% vs. 4.0%; HR: 0.85; 95% CI: 0.49-1.49; P = 0.57) between groups. Valve reintervention was required in 3.3% and 2.5% of patients in the TAVI and SAVR groups, respectively (HR 1.30, 95% CI 0.66-2.56, P = 0.44), while the incidence of clinical and subclinical valve thrombosis was low in both groups (0.3% vs. 0.2%, HR 1.84, 95% CI 0.17-20.24, P = 0.61, and 0.6% vs. 0.5%, HR 1.20, 95% CI 0.27-5.37, P = 0.81, respectively). The proportion of patients who were alive and well (alive and KCCQ summary score >75) were similar between the TAVI and surgery groups (70.6% and 69.3%, respectively).
The DEDICATE trial was an investigator-initiated RCT comparing SAVR with TAVI using any CE-marked devices in patients with severe symptomatic AS who were at low or intermediate surgical risk (N = 1,414, mean age of 74 years, STS-PROM 1.8%). The primary outcome was a composite of death from any cause or fatal or nonfatal stroke at 1 year. The Kaplan–Meier estimate of the primary outcome at 1 year was 5.4% in the TAVI group and 10.0% in the SAVR group (HR 0.53; 95% CI 0.35-0.79; Pnon-inferiority <0.001). The incidence of death from any cause was 2.6% in the TAVI group and 6.2% in the SAVR group (HR 0.43; 95% CI 0.24-0.73); the incidence of stroke was 2.9% and 4.7%, respectively (HR 0.61; 95% CI 0.35-1.06). At 1 year, TAVI was associated with an increased risk of new-onset conduction disturbances (left bundle branch block [LBBB]: 32.0% vs. 17.5%; HR 2.03; 95% CI 1.63-2.54; and permanent pacemaker implantation: 11.8% vs. 6.7%; HR 1.81; 95% CI 1.27-2.61, respectively) and vascular access-site complications (7.9% vs. 0.7%; HR 10.64; 95% CI 4.84-28.94), while SAVR was associated with a higher risk of new-onset atrial fibrillation (12.4% vs. 30.8%; HR 0.36; 95% CI 0.28-0.46) and major or life-threatening or disabling bleeding (4.3% vs. 17.2%; HR 0.24; 95% CI 0.16-0.35).
The NOTION-2 trial randomized 370 low-risk patients aged ≤75 years with severe symptomatic AS to TAVI or SAVR, including both tricuspid and bicuspid AS (mean age of 71.1 years and a median STS-PROM of 1.1%). The primary endpoint was a composite of all-cause mortality, stroke, or rehospitalization (related to the procedure, valve, or heart failure) at 12 months. The rate of the primary endpoint in the overall cohort was 10.2% in the TAVI group and 7.1% in the surgery group (absolute risk difference 3.1%; 95% CI -2.7% to -8.8%; HR 1.4; 95% CI, 0.7-2.9; P = 0.3). Patients undergoing TAVI had a lower risk of major or life-threatening bleeding (4.8% vs. 17.5%, HR 0.3, 95% CI 0.1-0.5) and new-onset atrial fibrillation (3.2% vs. 41.7%, HR 0.06, 95% CI 0.03-0.2) and a higher risk of non-disabling stroke (3.7% vs. 0.5%, HR 7.0, 95% CI 0.9-56.5), permanent pacemaker implantation (15.1% vs. 8.0%, HR 2.0, 95% CI 1.1-3.8), and moderate or greater PVR (4.7% vs. 0%, P = 0.005) compared to SAVR. The risk of the primary composite endpoint was similar in patients with tricuspid AS (8.7% in the TAVI group vs. 8.3% in the SAVR group, HR 1.0; 95% CI 0.5-2.3), while in patients with bicuspid AS, TAVI showed a trend towards a higher risk of the primary composite endpoint (14.3% vs. 3.9%, HR 3.8; 95% CI 0.8-18.5). Of note, the risk of stroke (4.1% vs. 0%, HR 4.1, 95% CI -1.5 to -9.6) and moderate or greater PVR (9.1% vs. 0%, HR 9.1, 95% CI 0.6-17.6) was more pronounced in bicuspid patients undergoing TAVI compared with SAVR.
The evidence base for TAVI is dominated by two landmark THV devices, the balloon-expandable SAPIEN and the self-expanding CoreValve/Evolut family of devices. In parallel, there have been prolific efforts to develop novel TAVI devices, which are being evaluated in direct head-to-head device comparisons. In this section, we review RCTs comparing TAVI devices which are relevant to inform device selection for individual patients (Table 8 and Figure 8).
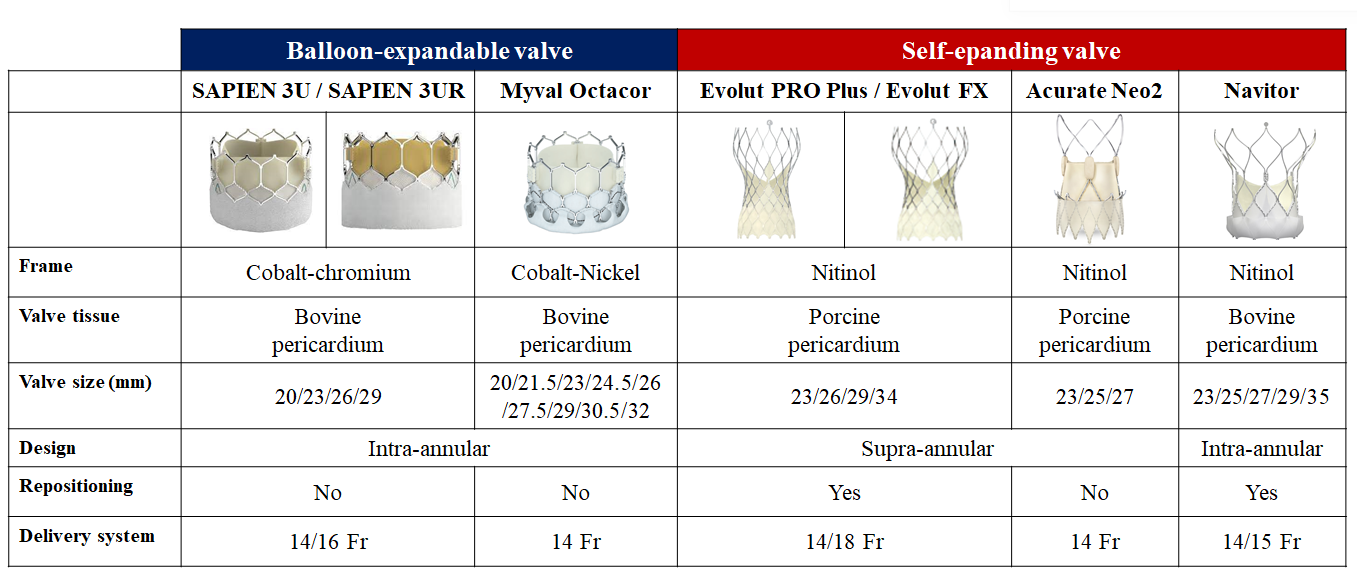
Contemporary transcatheter aortic valve implantation devices for the treatment of severe aortic stenosis.
Table 8. Device comparisons in randomized clinical trials.
|
Clinical Trial |
Study design |
N |
Valve performance at 30d |
PPI at 30d |
Primary endpoint |
Main result |
||
|---|---|---|---|---|---|---|---|---|
|
mPG (mmHg) |
≥mod PVR |
|||||||
|
CHOICE |
SAPIEN XT |
121 vs. 120 |
8.9 vs. 6.6 |
0% vs. 7.2% |
17.3% vs. 37.6% |
Device success as defined by the VARC criteria$ |
95.9% vs. 77.5% |
|
|
SCOPE I |
ACURATE neo |
372 vs. 367 |
7 vs. 11 |
9.4% vs. 2.8% |
10% vs. 9% |
Combination of two VARC-2-derived endpoints (early safety and clinical efficacy) at 30 days* |
24% vs. 16% |
|
|
PORTICO IDE |
Portico |
381 vs. 369 |
8.36 vs. 7.31 |
6.1% vs. 1.6% |
27.7% vs. 11.6% |
Safety endpoint: composite of all-cause mortality, disabling stroke, life-threatening or disabling bleeding requiring transfusion, acute kidney injury requiring dialysis, or major vascular complication at 30 days. |
13.8% vs. 9.6% |
|
|
Efficacy endpoint: composite of all-cause mortality or disabling stroke at 1 year. |
14.8% vs. 13.4% |
|||||||
|
SCOPE II |
ACURATE neo |
398 vs. 398 |
6.3 vs. 6.4 |
10% vs. 3% |
11% vs. 18% |
Powered for non-inferiority of the ACURATE neo THV, was the composite of all-cause death or stroke at 1 year |
15.8% vs. 13.9% |
|
|
SOLVE-TAVI |
Evolut R |
219 vs. 219 |
|
3.4% vs. 1.5% |
23.0% vs. 19.2% |
The efficacy composite endpoint of all-cause mortality, stroke, moderate/severe PVR, and permanent pacemaker implantation at 30 days |
28.4% vs. 26.1% |
|
|
LANDMARK |
Myval/Myval Octaor |
384 vs. 384 |
8.2 vs. 7.9 |
3% vs. 5% |
15% vs. 17% |
Composite of all-cause mortality, all stroke, bleeding (types 3 and 4), acute kidney injury (stages 2–4), major vascular complications, moderate or severe prosthetic valve regurgitation, and conduction system disturbances resulting in a permanent pacemaker implantation at 30 days |
25% vs. 27% |
|
|
ACURATE IDE |
Acurate Neo 2 |
752 vs. 748 |
8.0 vs. 12.0 (SAPIEN) or 7.0 (Evolut) |
1.1% vs. 0% (SAPIEN) or 0.8% (Evolut) |
12.0% vs.12.8% (HR 0.83, 95% CI 0.69-1.26) |
Composite of all-cause mortality, stroke, or rehospitalization (hospitalization for valve related symptoms or worsening congestive heart failure [NYHA class III or IV]; per VARC 2 definition) at 1 year |
16.2% vs. 9.5% (Posterior Median Difference and 95% BCI: 6.63% [3.04%, 10.2%]) |
|
|
COMPARE-TAVI 1 |
Myval/Myval Octaor |
514 vs. 517 |
9 vs. 11 |
2.2% vs. 0.6% |
19.3% vs. 10.5% |
Composite of all-cause mortality, stroke, moderate or severe aortic regurgitation, or moderate or severe haemodynamic valve deterioration at 1 year |
13.8% vs. 13.0% |
|
|
Reintervention: valve-related, procedural related dysfunction requiring repeat procedure Rehospitalization: valve-related, procedure-related, or heart failure-related rehospitalization. The results of SCOPE 1, PORTICO IDE, SCOPE 2, SOLVE-TAVI, LANDMARK, ACURATE IDE, and COMPARE-TAVI 1 trials are provided from intention-to-treat analyses. Blue indicates results with no significant difference between devices. Yellow indicates results with significant difference between devices. $: Composite endpoint including (1) successful vascular access, delivery, and deployment of the device and successful retrieval of the delivery system; (2) correct position of the device in the proper anatomical location; (3) intended performance of the prosthetic heart valve (AVA >1.2 cm2, mean aortic valve gradient <20 mmHg, or peak velocity <3 m/s, without moderate or severe prosthetic valve aortic regurgitation); and (4) only 1 valve implanted in the proper anatomical location. *: Composite of all-cause death, any stroke, life-threatening or disabling bleeding, major vascular complications, coronary artery obstruction requiring intervention, acute kidney injury (stage 2 or higher), rehospitalization for valve-related symptoms or congestive heart failure, valve-related dysfunction requiring repeat procedure, and valve-related dysfunction determined by echocardiography (mean aortic valve gradient ≥20 mm Hg and either effective orifice area ≤0.9–1.1 cm² [depending on body surface area] or Doppler velocity index <0.35; or moderate or severe prosthetic valve regurgitation as defined by VARC-2). mPG = mean transvalvular gradient; PVR = paravalvular regurgitation; TAVI = transcatheter aortic valve implantation; VARC = Valve Academic Research Consortium. |
||||||||
CHOICE was an investigator-initiated trial in high-risk patients with severe AS and an anatomy suitable for transfemoral TAVI. One hundred twenty-one patients were randomly assigned to receive a balloon-expandable SAPIEN XT device and 120 were assigned to receive a self-expandable CoreValve device. The primary endpoint was device success, a composite end point including successful vascular access and deployment of the device and retrieval of the delivery system, correct position of the device, intended performance of the heart valve without moderate or severe regurgitation, and only 1 valve implanted in the proper anatomical location. Device success was observed in 95.9% of patients in the balloon-expandable valve group and 77.5% of patients in the self-expandable valve group (relative risk 1.24, 95% CI 1.12-1.37, P <0.001), with differences attributed to a significantly lower frequency of residual more-than-mild aortic regurgitation (4.1% vs. 18.3%; relative risk 0.23; 95% CI 0.09-0.58; P <0.001) and the less frequent need for implanting more than 1 valve (0.8% vs. 5.8%, P = 0.03) in the balloon-expandable valve group. Clinical outcomes between groups were similar throughout 5-year follow-up, including all-cause death (53.4% vs. 47.6%, P = 0.38), cardiovascular death (31.6% vs. 21.5%, P = 0.12), all strokes (17.5% vs. 16.5%, P = 0.73), and repeat hospitalization for heart failure (28.9% vs. 22.5%, P = 0.75), while new pacemaker implantation was more frequent in the self-expanding group.
The SOLVE-TAVI trial was an investigator-initiated RCT of 447 patients with symptomatic severe AS undergoing transfemoral TAVI comparing the self-expanding Evolut R with the balloon-expandable SAPIEN 3 transcatheter valve systems. The primary efficacy composite endpoint included all-cause death, stroke, moderate or severe PVR, and new permanent pacemaker implantation at 30 days. The study was powered for equivalence of the primary endpoint (equivalence margin 10% with significance level 0.05). At 30 days, the primary endpoint occurred in 28.4% of the Evolut R arm and 26.1% of the SAPIEN 3 arm, meeting the prespecified criteria of equivalence (Pequivalence = 0.04). There was a numerically higher stroke rate in the SAPIEN arm (4.7% vs. 0.5%), while the rate of moderate or severe PVR was numerically higher in the Evolut R arm (3.4% vs. 1.5%). The rate of new permanent pacemaker implantation was higher than expected in both arms (23.0% vs. 19.2%). At 5 years, there was no significant difference in rates of the composite primary endpoint between the Evolut R and SAPIEN 3 arms (67.7% vs. 63.4%, P = 0.34), while stroke was more common in the SAPIEN 3 arm (2.2% vs. 9.6%, P = 0.002).
SCOPE I was an investigator-initiated RCT designed to compare the early safety and efficacy of the self-expanding ACURATE neo device to the balloon-expandable SAPIEN 3 system. In this trial, 739 patients (aged ≥75 years) with symptomatic severe AS undergoing transfemoral TAVI deemed at increased surgical risk were enrolled. The primary composite safety and efficacy endpoint comprised all-caused death, any stroke, life-threatening or disabling bleeding, major vascular complications, coronary artery obstruction requiring intervention, acute kidney injury (stage 2 or 3), rehospitalization for valve-related symptoms or congestive heart failure, valve-related dysfunction requiring repeat procedure, moderate or severe PVR, or prosthetic valve stenosis within 30 days of the procedure. The study was powered for non-inferiority of the ACURATE neo compared with the SAPIEN 3 THV for the primary endpoint (non-inferiority margin 7.7% with significance level 0.05). The primary endpoint occurred in 87 (24%) patients in the ACURATE neo and in 60 (16%) patients in the SAPIEN 3 group; non-inferiority of the ACURATE neo was not met (Pnon-inferiority = 0.42). The result was largely driven by a higher rate of acute kidney injury (3% vs. 1%, P = 0.034) and moderate or severe PVR (9% vs. 3%) in the ACURATE neo arm. In terms of haemodynamic outcomes, the ACURATE neo THV was associated with larger effective orifice area (1.73 cm2 vs. 1.47 cm2, P<0.001) and lower transvalvular gradients (7 mmHg vs. 11 mmHg, P <0.001) compared with the SAPIEN 3 THV. However, early differences between ACURATE neo and SAPIEN 3 did not translate into significant differences in clinical outcomes or bioprosthetic valve failure throughout 3 years of follow-up (all-cause death: 24.3% vs. 25.0%, HR 0.98, 95% CI 0.73-1.33; cardiovascular death: 16.8% vs. 16.8%, HR 1.01, 95% CI 0.70-1.45; stroke: 6.1% vs. 5.8%, HR 1.04, 95% CI 0.56-1.92; rehospitalization for valve-related symptoms or congestive heart failure: 13.9% vs. 18.1%, HR 0.74, 95% CI 0.51-1.07, new permanent pacemaker implantation: 15.6% vs. 16.4%, HR 0.92, 95% CI 0.62-1.37, respectively). Of note, the incidence of moderate or severe haemodynamic valve deterioration (HVD) according to the VARC-3 criteria (0.4% vs. 2.9%, subhazard ratios [sHR] 0.19, 95% CI 0.02-1.76) and valve thrombosis (0.3% vs. 1.8%, sHR 0.16, 95% CI 0.02-1.35) was numerically lower in the ACURATE neo group compared with that in the SAPIEN 3 group.
SCOPE II was another investigator-initiated RCT comparing the ACURATE neo to the Evolut R/PRO self-expanding valve system enrolling 796 patients (aged ≥75 years) with an indication for transfemoral TAVI. The primary endpoint, powered for non-inferiority of the ACURATE neo THV, was the composite of all-cause death or stroke at 1 year (non-inferiority margin 6% with significance level 0.05). The key secondary endpoint, powered for superiority of the ACURATE neo THV, was new permanent pacemaker implantation at 30 days. At 1 year, the primary endpoint occurred in 59 (15.8%) in the ACURATE neo and in 52 (13.9%) in the Evolut R/PRO group, with an absolute risk difference of 1.8% and a one-sided 95% upper confidence limit of 6.1% that did not meet non-inferiority of the ACURATE neo compared with the Evolut R/PRO THV (Pnon-inferiority = 0.0549). No significant differences were observed in the components of the primary endpoint. However, cardiac death at 30 days (2.8% vs. 0.8%, P = 0.03) and 1 year (8.4% vs. 3.9%, P = 0.01), and moderate or severe PVR (10% vs. 3%, P = 0.002) were higher in the ACURATE neo group. Conversely, the rate of new permanent pacemaker implantation was lower in the ACURATE neo than the Evolut R/PRO group both at 30 days (11% vs. 18%, P = 0.003) and 1 year (11% vs. 18%, P = 0.004). At 1 year, also new-onset LBBB occurred less frequently in the ACURATE neo compared with the Evolut R/PRO group (14% vs. 19%, P = 0.048).
Following the assessment of ACURTAE neo in SCOPE I and II, a new iteration - ACURATE neo 2 - with an annular sealing technology was developed which is used in clinical practice in Europe. The ACURATE IDE trial (NCT03735667) directly compared the ACURATE neo2 THV with the two established landmark devices (SAPIEN and Evolut). In this trial, 1,500 patients with tricuspid severe symptomatic AS were randomly assigned 1:1 to ACURATE neo 2 (N = 752) or the control group (SAPIEN [N = 504] or Evolut [N = 244]). The primary hypothesis was non-inferiority of ACURATE neo 2 to the control group for the primary endpoint of all-cause mortality, stroke, or hospitalization for valve-related symptoms or worsening congestive heart failure at 1 year. At 1 year, the primary endpoint occurred in 16.2% (95% BCI 13.4% to 19.1%) of patients receiving ACURATE neo 2 and in 9.5% (95% BCI 7.5% to 11.9%) of patients receiving SAPIEN/Evolut, with between-group difference of 6.6% (95% BCI, 3.0% to 10.2%) and posterior probability of treatment difference of >0.999, failing to establish non-inferiority of ACURATE neo 2 compared to the contemporary devices. Patients in the ACURATE neo 2 group had a higher rate of cardiovascular death, stroke, and spontaneous myocardial infarction than those in the control groups, while clinically evident valve thrombosis occurred less frequently with ACURATE neo 2 than with control valves (0.7% vs. 2.6%, HR 0.26, 95% CI 0.10-0.71) and this difference was more pronounced when compared with the SAPIEN device (clinically evident valve thrombosis: 3.3% in the SAPIEN group and 1.3% in the Evolut group, respectively). A post-hoc analysis suggested that valve under-expansion may have had an impact on 1-year clinical outcomes. More than 1 in 5 patients assigned to ACURATE neo 2 had evidence of under-expanded valve frames, defined as non-parallel commissural posts on angiographic imaging, and under-expansion was associated with an increased risk of the primary endpoint (primary endpoint: 18.8% vs. 12.4%, P = 0.050; death: 7.4% vs. 3.7%, P = 0.054; stroke: 11.0% vs. 3.5%, P <0.001; rehospitalization: 2.7% vs. 5.9%, P = 0.131; respectively). Patients with well-expanded ACURATE neo2 valves had a similar rate of death and stroke at 1 year compared to controls in this post-hoc analysis (3.7% vs. 3.6% and 3.5% vs. 3.4%, respectively).
PORTICO IDE was a non-inferiority RCT to evaluate the safety and efficacy of the Portico intra-annular self-expanding THV compared with any commercially available THV (SAPIEN: 1.4%, SAPIEN XT: 7.2%, SAPIEN 3: 57.1%, CoreValve: 3.9%, Evolut R: 24.7%, and Evolut PRO: 5.8%). The primary safety endpoint was a composite of all-cause death, disabling stroke, life-threatening bleeding requiring transfusion, acute kidney injury requiring dialysis, or major vascular complication at 30 days. The primary efficacy endpoint was all-cause death or disabling stroke at 1 year. The non-inferiority margin was 8.5% for primary safety and 8.0% for primary efficacy endpoints. The Portico THV met non-inferiority criteria for the primary safety endpoint, which occurred in 13.8% of the Portico and in 9.6% of the commercial valve group (Pnon-inferiority = 0.034). At 1 year, the Portico THV also met non-inferiority criteria for the primary efficacy endpoint (14.8% vs. 13.4%, Pnon-inferiority = 0.006). At 2 years, rates of all-cause death (22.3% vs. 20.2%, P = 0.40) or disabling stroke (3.1% vs. 5.0%, P = 0.32) were similar between groups. In terms of valve performance, the Portico THV was associated with similar mean transvalvular gradients (8.1 mmHg vs. 7.4 mmHg, P = 0.18) and effective orifice area (1.86 cm2 vs. 1.76 cm2, P = 0.16) as the Evolut R/PRO THV, and lower gradients and larger effective orifice area than the SAPIEN 3 THV (11.5 mmHg, P <0.001; and 1.62 cm2, P <0.001; respectively). Conversely, moderate or severe PVR occurred more frequently in the Portico group than in the SAPIEN 3 group (6.1% vs. 1.6%, P = 0.016), but no difference was observed when compared with the Evolut R/PRO group (4.0%, P = 0.42).
The ENVISION trial (NCT05932615) is currently underway to compare the Navitor THV, a successor to the Portico THV, to the established landmark devices in a head-to-head randomized comparison.
The LANDMARK trial was a prospective, multinational, randomized, open-label, non-inferiority trial that compared the balloon-expandable Myval THV with the contemporary SAPIEN or Evolut series. The Myval THV is characterized by smaller sizing increments of 1.5 mm compared with the usual 3 mm increments in valve size. Out of 5,109 screened patients, 768 patients deemed eligible for all three devices were randomly assigned 1:1 to Myval (N = 384) or contemporary THV (N = 384) with subsequent stratification and equal allocation (1:1) of patients to the SAPIEN (N = 192 [SAPIEN 3: 55.4% and SAPIEN 3 Ultra: 44.6%]) or Evolut (N = 192 [Evolut R: 37.0%, Evolut PRO: 55.2%, Evolut PRO Plus: 5.2%, and Evolut FX: 2.6%]) devices using a covariate-adaptive randomization process. The primary early safety and effectiveness composite endpoint at 30-day follow-up included all-cause mortality, stroke, bleeding type 3 & 4, acute kidney injury stage 2-4, moderate or severe PVR, new permanent pacemaker implantation, or major vascular complications. The study was powered for non-inferiority of the primary endpoint (non-inferiority margin 10.44% at a significance level of 0.05). The trial established non-inferiority of the Myval THV (25%) compared with contemporary THV (27%) for the primary endpoint with a risk difference of -2.3% (one-sided upper 95% CI 3.8, Pnon-inferiority <0.0001). There were no significant differences for the individual components of the primary composite endpoint. For the individual head-to-head comparisons, this trial performed a prespecified analysis assuming an event rate of 26.1% for the primary endpoint and a non-inferiority margin of 10.4% the sample size provided 80% power at a one-sided alpha of 0.05 to establish non-inferiority. At 30 days, Myval was found to be non-inferior for the composite primary endpoint as compared to each comparator device (24.7% vs. 24.1% for SAPIEN; risk difference: 0.6%, one-sided upper 95% CI: 8.0%, Pnon-inferiority: 0.0033; and 24.7% vs. 30.0% for Evolut; risk difference: -5.3%, one-sided upper 95% CI: 2.5%, Pnon-inferiority: <0.0001). In terms of echocardiographic outcomes as assessed at an independent Core Laboratory, Myval was associated with lower mean transprosthetic gradients and larger effective orifice area and a similar rate of moderate or greater prosthetic valve regurgitation compared to SAPIEN. As compared with Evolut, Myval was associated with higher mean transprosthetic gradients and smaller effective orifice area and a lower rate of moderate or greater prosthetic valve regurgitation. However, a similar rate of moderate or greater prosthetic valve regurgitation was observed for Myval and Evolut THV after excluding Evolut R prostheses.
The COMPARE-TAVI 1 trial was an investigator-initiated, all-comers, non-inferiority trial comparing two balloon-expandable THV Out of 1,335 patients undergoing transfemoral TAVI at three high-volume centers in Western Denmark (defined as more than 75 TAVI procedures per year), 1,031 patients were randomized 1:1 to treatment with SAPIEN 3 (29 mm) / SAPIEN 3 Ultra (20, 23, or 26 mm) THV or Myval/Myval Octacor THV (median age: 81 years; female: 40.3%; median STS-PROM: 2.3%; bicuspid anatomy: 9.5%; and valve-in-valve procedure: 4.0%). The primary endpoint was a composite of all-cause death, stroke, moderate or severe aortic regurgitation, or moderate or severe HVD at 1 year according to the VARC-3 criteria. The non-inferiority margin was adjusted based on the actual event rate, and a non-inferiority margin of 5.3% with an upper limit of one-sided 95% CI of 4.4% was selected. At 1 year, the primary composite endpoint occurred in 13.0% of patients treated with SAPIEN THV and 13.8% of patients treated with Myval THV, demonstrating non-inferiority of Myval THV to SAPIEN THV (risk difference -0.9%, one-sided upper 95% CI -4.4%, Pnon-inferiority = 0.02). For the components of the primary endpoint, there was no significant difference in the rates of mortality, stroke, and moderate or severe HVD, while moderate or severe aortic regurgitation occurred more frequently in the Myval group compared with the SAPIEN group (3.9% vs. 1.2%, P = 0.005). In addition, the Myval group had a higher incidence of new permanent pacemaker implantation (20.9% vs. 12.0%, P <0.001), newly diagnosed atrial fibrillation (13.5% vs. 8.1%, P = 0.003) and VARC-3 type 2 bleeding (14.8% vs. 7.9%, P <0.001) compared with the SAPIEN group. However, Myval THV had a larger effective orifice area and smaller transprosthetic gradient across all THV sizes and across the total range of annulus areas. Of note, there was a wide range in the rate of predilatation (Myval: 20.2%, Myval Octacor: 59.0%, and SAPIEN: 20.7%, respectively), which may influence valve haemodynamics and the occurrence of conduction disturbances. A prespecified CT substudy is currently underway to evaluate the relationship between actual valve deployment and valve performance or the occurrence of conduction disturbances.
Following a series of RCTs demonstrating similar clinical outcomes with the less invasive TAVI intervention compared with SAVR, current ACC/AHA and ESC/EACTS guidelines for the management of valvular heart disease recommend TAVI as an alternative to SAVR across the spectrum of surgical risk among elderly patients who are candidates for bioprostheses (Figure 7), . Although most of the evidence supporting TAVI compared with SAVR has been generated in carefully selected patient populations with predominantly two valve types, the favourable outcomes of TAVI have been corroborated in investigator-initiated trials with broader inclusion criteria (NOTION, NOTION-2, DEDICATE, UK-TAVI) more closely resembling clinical practice. Notwithstanding, each treatment strategy has its own strengths and limitations, which should be considered by interdisciplinary Heart Teams to provide the best possible care for each individual patient. The following sections highlight key considerations that should be taken into account to inform the individual decision between TAVI and SAVR (Table 9).
Table 9. A framework of factors favouring SAVR, TAVI, or conservative therapy (2020 ACC/AHA guideline).
|
|
Favours SAVR |
Favours TAVI |
Favors Conservative Therapy |
|---|---|---|---|
|
Age/life expectancy |
-Younger age/longer life expectancy |
-Older age/fewer expected remaining years of life |
-Limited life expectancy |
|
Valve morphology |
-Bicuspid aortic valve -Left ventricular outflow tract calcification -Rheumatic valve disease -Small or large aortic annulus |
-Calcific AS of a tri-leaflet valve |
|
|
Prosthetic valve preference |
-Mechanical or surgical bioprosthetic valve preferred -Concern for prosthesis–patient mismatch (annular enlargement might be considered) |
-Bioprosthetic valve preferred -Favourable ratio of life expectancy to valve durability -TAVI provides larger valve area than same size SAVR |
|
|
Concurrent cardiac conditions |
-Aortic dilation -Severe primary mitral regurgitation -Severe coronary artery disease requiring bypass grafting -Septal hypertrophy requiring myectomy -Atrial fibrillation |
-Severe calcification of the ascending aorta (porcelain aorta) |
-Irreversible severe left ventricular systolic dysfunction -Severe mitral regurgitation attributable to annular calcification |
|
Non-cardiac conditions |
|
-Severe lung, liver, or renal disease -Mobility issues (high procedural risk with sternotomy) |
-Symptoms likely attributable to noncardiac conditions -Severe dementia -Moderate to severe involvement of ≥2 other organ systems |
|
Frailty |
-No or little frailty |
-Likely to improve after TAVI |
-Unlikely to improve after TAVI |
|
Estimated procedural or surgical risk of AVR |
-SAVR risk low -TAVI risk high |
-TAVI risk low to medium -SAVR risk high to prohibitive |
-Prohibitive SAVR risk (>15%) or post-TAVI life expectancy <1y |
|
Procedure-specific impediments |
-Valve anatomy, annular size, or low coronary ostial height precludes TAVI -Vascular access does not allow transfemoral TAVI
|
-Previous cardiac surgery with at-risk coronary grafts -Previous chest irradiation
|
-Valve anatomy, annular size, or coronary ostial height precludes TAVI -Vascular access does not allow transfemoral TAVI |
|
Goals of Care and patient preferences and values |
-Less uncertainty about valve durability -Avoid repeat intervention -Lower risk of permanent pacer -Life prolongation -Symptom relief -Improved long-term exercise capacity and QOL -Avoid vascular complications -Accepts longer hospital stay, pain in recovery period |
-Accepts uncertainty about valve durability and possible repeat intervention -Higher risk of permanent pacer -Life prolongation -Symptom relief -Improved exercise capacity and QOL -Prefers shorter hospital stay, less postprocedural pain |
-Life prolongation not an important goal -Avoid futile or unnecessary diagnostic or therapeutic procedures -Avoid procedural stroke risk -Avoid possibility of cardiac pacer |
|
ACC/AHA = American College of Cardiology/American Heart Association; AS = aortic stenosis; AVR = aortic valve replacement; QOL = quality of life; SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation. |
|||
Current guidelines for the management of valvular heart disease recommend TAVI in patients with severe AS aged ≥65 years (ACC/AHA guidelines) or ≥75 years (ESC/EACTS guidelines), . Although most patients undergoing TAVI or SAVR are >75 years of age, a non-negligible proportion of patients require intervention before 70 years of age. As TAVI is expanding to younger patients with longer life-expectancy, it is important to anticipate lifetime management issues beyond the first 10 years after the index procedure, namely the need for reintervention. The available evidence comparing TAVI with SAVR is limited to 5-10 years with the majority of participants aged >70 years, even in the low-risk trials (PARTNER 3: mean age of 73 years, Evolut Low Risk: mean age of 74 years), , , , , , , , . The NOTION-2 trial compared TAVI with SAVR exclusively in patients aged ≤75 years (mean age of 71.1 years and a median STS-PROM of 1.1%) and reported a similar incidence of the primary composite endpoint of all-cause mortality, stroke, or rehospitalization between the treatment strategies. However, the follow-up period is limited to 1-year so far. Although previous surgical studies reported that clinically relevant deterioration of surgical bioprostheses typically occurs after >10 years, data on durability of THV is currently limited up to 10 years, as will be discussed in a later section.
Previous surgical studies have suggested that women compared to men have impaired clinical outcomes due to later disease presentation and more advanced comorbid status at the time of intervention, . Early data from the STS/ACC TVT registry (11,808 women and 11,844 men between 2011 and 2014) reported more procedural complications including major vascular complications, VARC-2 major bleeding, and more frequent surgical conversion in female than male patients, while female patients had a lower 1-year all-cause death compared with male patients (adjusted HR 0.73, 95% CI 0.63-0.85). Of note, more than 30% of patients in this study underwent non-transfemoral TAVI and all TAVI devices adopted a large bore sheath (>20 Fr). Due to technical and device improvements, increased operator experience, and improved patient selection, sex-specific differences in clinical outcomes have been less pronounced. In the SOURCE 3 registry of 1,649 patients undergoing transfemoral TAVI with the balloon-expandable SAPIEN3 device (49.2% women, age 81.7 ± 6.7 years), no sex-related difference was observed in terms of procedural outcomes and mortality throughout 4-year follow-up.
Several studies have suggested that TAVI may be more effective than SAVR in female patients with severe AS. However, data were limited to subgroup analyses from registry or RCTs, , . The RHEIA trial was the first trial directly comparing SAVR with TAVI in female patients with symptomatic severe AS. In this study, 443 female patients with symptomatic severe AS (mean age 73 years and mean STS-PROM 2%) were randomly assigned to SAVR or TAVI with the SAPIEN 3 or SAPIEN 3 Ultra THV. At 1 year, the primary composite endpoint of all-cause mortality, stroke, and rehospitalization for valve- or procedure-related symptoms or worsening heart failure was higher in the SAVR compared with the TAVI group (15.6% vs. 8.9%, difference -6.8%, upper 95% confidence limit -1.5%, Pnon-inferiority <0.001, two-sided 95% CI -13.0% to -0.5%, Psuperiority = 0.034), predominantly driven by a higher rehospitalization rate in the SAVR group (11.4% vs. 4.8%) without differences in terms of mortality (2.0% vs. 0.9%) and stroke (3.0% vs. 3.3%). Although the majority of the study participates represented with small aortic annulus dimensions defined as aortic annulus area <430 mm, favourable bioprosthetic haemodynamics were achieved with both treatment approaches in this population.
A considerable proportion of patients with clinically relevant AS have relevant comorbidities when referred for AVR. These factors must be taken into careful consideration during the decision-making process on treatment selection. Current ACC/AHA guidelines indicate high or prohibitive surgical risk when patients have one or more major organ systems compromised and not to be improved postoperatively (Table 10).
Table 10. Risk assessment for surgical aortic valve replacement (2020 ACC/AHA guideline)
| Criteria | Low surgical risk (must meet all criteria in this column) |
High surgical risk (any 1 criterion in this column) |
Prohibitive surgical risk (any 1 criterion in this column) |
|||
|---|---|---|---|---|---|---|
| STS-PROM | < 3% | > 8% | Predicted risk of death or major morbidity (all-cause) > 50% at 1 y | |||
| Frailty+ | None | ≥ 2 indices (moderate to severe) | ≥ 2 indices (moderate to severe) | |||
| Major organ system compromise not to be improved postoperatively++ | None | 1 to 2 organ systems | ≥ 3 organ systems | |||
| Procedure-specific impediment+++ | None | Possible procedure-specific impediment | Severe procedure-specific impediment | |||
| + Seven frailty indices: Katz Activities of Daily Living (independence in feeding, bathing, dressing, transferring, toileting, and urinary continence) plus independence in ambulation (no walking aid or assistance required, or completion of a 5-m walk in < 6 s). Other scoring systems can be applied to calculate no, mild, or moderate to severe frailty. ++ Examples of major organ system compromise include cardiac dysfunction (severe left ventricular systolic or diastolic dysfunction or right ventricular dysfunction, fixed pulmonary hypertension); kidney dysfunction (chronic kidney disease, stage 3 or worse); pulmonary dysfunction (FEV1 < 50% or DLCO2 < 50% of predicted); central nervous system dysfunction (dementia, Alzheimer’s disease, Parkinson’s disease, cerebrovascular accident with persistent physical limitation); gastrointestinal dysfunction (Crohn’s disease, ulcerative colitis, nutritional impairment, or serum albumin < 3.0); cancer (active malignancy); and liver dysfunction (any history of cirrhosis, variceal bleeding, or elevated international normalized ratio in the absence of vitamin K antagonist therapy). +++ Examples of procedure-specific impediments include presence of tracheostomy, heavily calcified (porcelain) ascending aorta, chest malformation, arterial coronary graft adherent to posterior chest wall, and radiation damage. ACC/AHA = American College of Cardiology/American Heart Association; DLCO2 = diffusion capacity for carbon dioxide; FEV1 = forced expiratory volume in 1 second; STS-PROM = Society of Thoracic Surgeons Predicted Risk of Mortality. |
||||||
Although RCTs excluded highly morbid patients, TAVI is more attractive than SAVR in this population as long as life expectancy exceeds 1 year due to the less invasive nature of the intervention and faster recovery, . However, patients with severe morbidities, such as end-stage renal disease, end-stage liver disease, chronic obstructive pulmonary disease, and advanced cancer, have worse prognosis compared with those without, even after successful TAVI and discussion regarding futility may be considered, , , .
Frailty is a multidimensional geriatric syndrome defined as slowness, weakness, exhaustion, wasting and malnutrition, poor endurance and inactivity, and loss of independence. The prevalence of frailty in patients undergoing AVR varies widely between studies due to differences in patient populations and varying definitions (Table 11).
Table 11. Frailty Assessment.
|
Parameters |
Tests |
Scales |
||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
Fried |
Fried+ |
SPPB |
Rockwood |
Bern |
Columbia |
EFT |
|
Slow gait speed |
Time to walk 5 meters at a comfortable pace (3 trials) |
✓ |
✓ |
✓ |
|
✓ |
✓ |
|
|
Upper-extremity weakness |
Dynamometer-measured maximal grip strength (3 trials) |
✓ |
✓ |
|
|
|
✓ |
|
|
Lower-extremity weakness |
Time to stand 5 times from a chair without using arms |
|
|
✓ |
|
|
|
✓ |
|
Poor balance |
Time able to stand with feet in tandem or side-by-side positions (up to 10 sec) |
|
|
✓ |
|
|
|
|
|
Weight loss |
Self-reported unintentional weight loss ≥10 lbs over the past year |
✓ |
✓ |
|
|
|
|
|
|
Exhaustion |
Self-reported “could not get going” or “everything was an effort” most days of the week |
✓ |
✓ |
|
✓ |
|
|
|
|
Inactivity |
Paffenbarger questionnaire (expanded version) <2000 kilocalories of weekly activity |
✓ |
✓ |
|
✓ |
|
|
|
|
Anaemia |
Haemoglobin <13.0 g/dL in men and <12.0 g/dL in women |
|
|
|
|
|
|
✓ |
|
Hypoalbuminemia |
Serum albumin <3.5 g/dL in men and women |
|
|
|
|
|
✓ |
✓ |
|
Malnutrition |
Mini-Nutritional Assessment |
|
|
|
|
✓ |
|
|
|
Cognitive impairment |
Mini-Mental State Examination |
|
✓ |
|
|
✓ |
|
✓ |
|
Depressed mood |
Short-Form Geriatric Depression Scale |
|
✓ |
|
|
|
|
|
|
ADL disability |
Inability to independently perform basic activities such as eating and dressing |
|
|
|
✓ |
✓ |
✓ |
|
|
IADL disability
|
Inability to independently perform instrumental activities such as cooking and shopping |
|
|
|
✓ |
✓ |
|
|
|
ADL = Activities of Daily Living; EFT = Essential Frailty Toolset; IADL = Instrumental Activities of Daily Living; SPPB = Short Physical Performance Battery. |
||||||||
The prospective FRAILTY-AVR study assessed frailty in patients with severe AS undergoing TAVR or SAVR using 7 different frailty scales and observed that prevalence ranged from 35-74% in the TAVR group and 12-56% in the SAVR group, depending on the definition used. The Essential Frailty Toolset, integrating lower-extremity weakness, cognitive impairment, anaemia, and hypoalbuminemia, outperformed the other scales and was recommended for use in this setting. Despite varying prevalence, frailty has been consistently associated with worse health status and overall prognosis compared to non-frail patients after AVR, , , , . A significant proportion of frail patients can achieve a similar extent of improvement in patient-reported outcome as non-frail patients, which may justify invasive treatment strategies in patients with reduced health status and frailty at baseline, even in the absence of an effect on mortality. In a post-hoc analysis of the SCOPE I trial, patients with frailty demonstrated similar improvement in KCCQ overall score after TAVI, while frailty was associated with an increased risk of VARC-3 unfavourable outcomes throughout 3 years of follow-up (48.0% vs. 34.7%, risk ratio 1.38, 95% CI 1.09-1.75), suggesting that TAVI may be futile in some frail patients. Current guidelines allocate frailty as key consideration in the decision-making process between SAVR and TAVI (Table 10). However, recent analyses from the PARTNER trials and pooled outcomes of the CoreValve High Risk and SURTAVI trials suggest that frailty is not associated with a differential impact with respect to treatment modality on mortality and disease-specific health status, . Furthermore, frailty status may improve with initial relief of mechanical obstruction and improved patient care during subsequent follow-up. The PERFORM-TAVR trial randomized elder frail patients undergoing TAVI to protein oral supplementation and exercise therapy (85 patients) or usual care (95 patients). At 3 months after TAVR, patients in the intervention group demonstrated better physical performance compared to the control group by an improvement of 1.02 points in the SPPB score. Of note, a nationwide Danish study reported an important and ongoing increase in frailty and comorbidity burden from the time of TAVI to the end of life in TAVI recipients. These results underline the importance of a comprehensive approach to all phases of care for elder frail patients undergoing TAVI.
Surgical risk is usually assessed using STS-PROM and EuroSCORE II, . According to these scores, patients have been stratified into three risk categories: high (>8%), intermediate (4%-8%), and low (<4%) surgical risk. Of note, the risk scores have been developed in cohorts of patients undergoing cardiac surgery, and their predictive performances in TAVI population have been limited. Several TAVI-specific risk prediction models have been investigated, , , , , . However, their predictive performance was not superior to conventional surgical risk scores (Table 12), maintaining STS-PROM and EuroSCORE II as preferred risk prediction models in current guidelines, .
Table 12. Risk Scores.
|
Criterion |
ES I |
ES II |
STS |
FRANCE-2 |
OBSERVANT |
STS/ACC TVT |
German AV Score II |
|---|---|---|---|---|---|---|---|
|
Publication year |
1999/2003 |
2012 |
2009 |
2014 |
2014 |
2016 |
2017 |
|
Cohort size (Development/Validation) |
13,302/1,479 |
16,828/5,553 |
65,855/43,904 |
2,552/1,281 |
1,256/622 |
13,718/6,868 |
9,027/9,027 |
|
Procedure |
Cardiac surgery |
Cardiac surgery |
Cardiac valve surgery |
TAVI |
TAVI |
TAVI |
55% SAVR 45% TAVI |
|
Discrimination performance (C statics in validation cohort) |
0.76 |
0.81 |
0.80 |
0.59 |
0.71 |
0.66 |
0.74 |
|
Risk factor |
|
|
|
|
|
|
|
|
Age |
✓ |
✓ |
✓ |
✓ |
|
✓ |
✓ |
|
Sex |
✓ |
✓ |
✓ |
|
|
✓ |
✓ |
|
Body physique |
|
|
✓ |
✓ |
|
|
✓ |
|
Race |
|
|
✓ |
|
|
✓ |
|
|
Left ventricular function |
✓ |
✓ |
✓ |
|
✓ |
|
✓ |
|
Cardiac symptoms |
✓ |
✓ |
✓ |
|
|
|
✓ |
|
Myocardial infarction |
✓ |
✓ |
✓ |
|
|
|
|
|
NYHA class |
|
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
|
Rhythm |
|
|
✓ |
|
|
|
✓ |
|
Hypertension |
|
|
✓ |
|
|
|
|
|
Endocarditis |
✓ |
✓ |
✓ |
|
|
|
✓ |
|
Coronary artery disease |
|
|
✓ |
|
|
|
✓ |
|
Previous cardiac surgery |
✓ |
✓ |
✓ |
|
|
|
✓ |
|
Tricuspid valve disease |
|
|
✓ |
|
|
|
|
|
Mitral valve disease |
|
|
✓ |
|
|
|
|
|
Chronic lung disease |
✓ |
✓ |
✓ |
✓ |
|
✓ |
✓ |
|
Pulmonary hypertension |
✓ |
✓ |
|
✓ |
✓ |
|
✓ |
|
Cerebrovascular disease |
✓ |
|
✓ |
|
|
|
|
|
Peripheral artery disease |
✓ |
✓ |
✓ |
|
|
|
✓ |
|
Renal function |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
|
Diabetes |
|
✓ |
✓ |
|
✓ |
|
✓ |
|
Mobility |
|
✓ |
|
|
|
|
|
|
Immunocompromise |
|
|
✓ |
|
|
|
|
|
Status |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
|
Previous balloon aortic valvuloplasty |
|
|
|
|
✓ |
|
|
|
Approach |
|
|
|
✓ |
|
✓ |
|
|
Pulmonary oedemas during the last year |
|
|
|
✓ |
|
|
|
|
ES I = EuroSCORE I; ES II = EuroSCORE II; German AV Score II = German Aortic Valve Score II; STS = Society of Thoracic Surgeons score; STS/ACC TVT = Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry TAVI in-hospital mortality risk score. COPD = chronic obstructive pulmonary disease; NYHA = New York Heart Association; SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation. |
|||||||
More recently, artificial intelligence-based predictive models for TAVI outcomes have been developed. The TAVI Risk Machine (TRIM) scores is a machine learning-based model for predicting 30-day survival after TAVI. The algorithm was trained and cross-validated on data from 22,283 patients within the nationwide GARY registry and validated on data from 6,693 patients within the nationwide SwissTAVI registry. The model outperformed STS-PROM (TRIM: C-statistic 0.79, 95% CI 0.74-0.83; STS-PROM: C-statistic 0.69, 95% CI 0.65-0.74 in the deviation set and TRIM: C-statistic 0.75, 95% CI 0.72-0.79; STS-PROM: C-statistic 0.67, 95% CI 0.63-0.70 in the validation set).
AS and coronary artery disease (CAD) often coexist and share pathophysiological mechanisms. Therefore, screening for significant CAD prior to AVR is important to determine the need for coronary revascularization. Current ACC/AHA and ESC/EACTS guidelines recommend invasive coronary angiography to screen for CAD and limit coronary computed tomography angiography (CTA) to exclude obstructive CAD in patients with a low pretest probability of CAD. However, CTA is increasingly used to simultaneously assess the valvular apparatus and exclude significant stenoses of the proximal coronary artery segments, circumventing the need for invasive coronary angiography, , .
While SAVR and concomitant coronary artery bypass grafting has been the traditional and established standard of care for patients with AS and significant CAD, a complete percutaneous strategy (i.e. TAVI and percutaneous coronary intervention [PCI]) has been suggested as a reasonable treatment alternative in this population. However, the prognostic impact of concomitant CAD and the role of PCI remain unresolved in TAVI population, largely related to varying definitions of CAD. In the NOTION-3 trial, 455 patients (median age of 82 years; median STS-PROM 3.0) with at least one high-grade coronary artery stenosis defined as fractional flow reserve ≤0.80 or diameter stenosis ≥90% in a coronary artery ≥2.5 mm diameter were randomly assigned to PCI with complete revascularization or to conservative treatment. The majority of PCI procedures were performed prior to TAVI and complete revascularization was achieved in 89% of patients in the PCI group. At a median follow-up of 2 years, the primary composite endpoint of death from any cause, myocardial infarction, or urgent revascularization was lower in the PCI group than in the conservative treatment group (26% vs. 36%, P = 0.04), driven by lower rates of spontaneous myocardial infarction or urgent revascularization. Of note, the benefit of PCI on adverse cardiac events was more pronounced among patients with ≥90% diameter stenosis as compared to fractional flow reserve <0.80.
TAVI has the advantage of deferring treatment of concomitant CAD, allowing treatment to be prioritized according to disease severity, complexity and clinical presentation. PCI before TAVI has been proposed to reduce ischemic adverse events and to facilitate coronary access. However routine PCI prior to TAVI may be associated with an increased risk of periprocedural adverse events. Staged PCI after TAVI may provide advantages in terms of procedural safety and adequate physiological assessment of coronary lesion severity, , . A TAVI-first approach followed by deferred PCI as necessary may emerge as strategy in TAVI patients without high-grade CAD (i.e., 70-90% stenosis) (Figure 9). Ongoing trials will provide further perspectives into the optimal management strategy in patients with severe AS and CAD referred to TAVI (Table 13).
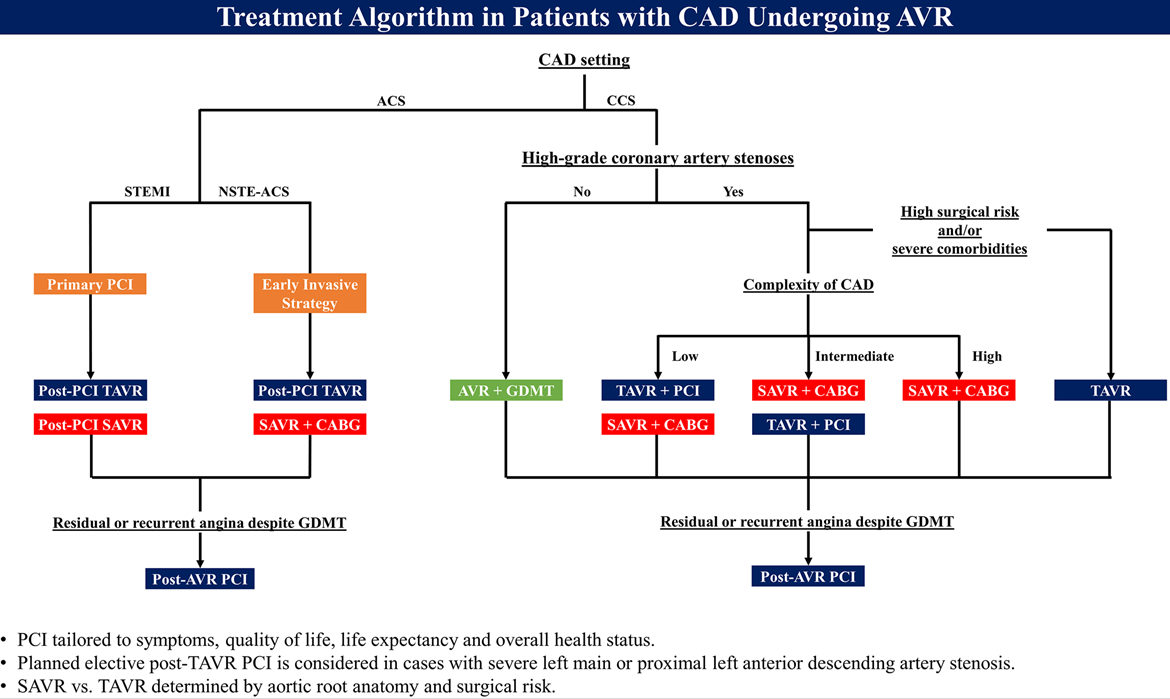
Treatment algorithm in patients with significant coronary artery disease undergoing aortic valve replacement.
Figures reproduced from Tomii et al. Circulation 2024;150(25):2046-2069.
Table 13. Ongoing randomized clinical trials in patients with aortic stenosis and coronary artery disease undergoing TAVI.
|
Clinical Trial |
N |
Intervention |
CAD definition |
Primary outcome |
Follow-up period |
Estimated Completion |
|---|---|---|---|---|---|---|
|
Complete revascularization |
||||||
|
COMPLETE-TAVR |
4000 |
FFR-guided PCI with complete revascularization |
At least one coronary artery lesion with ≥70% visual angiographic diameter stenosis in a native segment ≥2.5 mm in diameter |
Composite of cardiovascular death or new myocardial infarction or ischemia-driven revascularization or hospitalization for unstable angina or heart failure |
Median follow-up of 3.5 years |
2026 |
|
Mode of PCI |
||||||
|
FAITAVI |
320 |
Physiologically-guided PCI |
Coronary stenosis ≥50% by visual assessment in vessels ≥2.5 mm |
Composite of all-cause death, myocardial infarction, stroke, major bleeding, need for target vessel revascularization |
1 year |
2024 |
|
Lesions with FFR ≤0.80 |
||||||
|
Timing of PCI |
||||||
|
TAVI-PCI |
986 |
PCI before TAVI |
Any suitable lesion with ≥70% diameter stenosis on coronary angiography in a coronary artery ≥2.5 mm in diameter |
Composite of all-cause death, non-fatal myocardial infarction, ischemia-driven revascularization, rehospitalization for valve- or procedure-related including heart failure, life-threatening/disabling or major bleeding according to VARC-2 criteria |
1 year |
2026 |
|
Need routine PCI / routine coronary artery assessment |
||||||
|
PRO-TAVI |
466 |
TAVI without routine PCI |
At least 1 stenosis (70-99% diameter stenosis in angiography or 40-70% diameter stenosis in angiography with positive haemodynamic parameters) in epicardial coronary artery (>2.5 mm) or bypass graft |
Composite of all-cause mortality, myocardial infarction, stroke and type 2-4 bleeding, in accordance to VARC-3 criteria |
1 year |
2025 |
|
CAT |
546 |
No routine angiographic coronary artery assessment |
PCI recommended for coronary diameter stenosis of ≥ 80% in coronary segments with a reference vessel diameter of at least 2.5 mm. |
Composite of all-cause death, myocardial infarction, any stroke and heart failure hospitalization (VARC-3 criteria) |
3 years |
2029 |
|
AS = aortic stenosis; CAD = coronary artery disease; FFR = fractional flow reserve; PCI = percutaneous coronary intervention; TAVI = transcatheter aortic valve implantation; VARC = Valve Academic Research Consortium. |
||||||
The presence of moderate or severe mitral regurgitation and tricuspid regurgitation and clinically significant mitral stenosis (mitral valve area ≤1.5 cm2) is not infrequently observed in patients with severe AS and associated with an increased risk of adverse events even after AVR, , . Therefore, the presence of concomitant valvular heart disease should be carefully evaluated before the intervention (Figure 10). However, diagnosis of multivalvular disease is challenging since the haemodynamic interdependency between primary and secondary valvular lesions complicates the assessment of the true severity of each valvular lesion. Similarly, treatment strategy and timing of interventions are complex due to lack of prospective studies and uncertainty of best clinical practice. For patients with severe AS and significant primary valvular heart disease, SAVR and concomitant surgical valve repair/replacement is the first-line therapy unless the surgical risk is not high. In turn, secondary/functional valvular disease is more likely to regress after aortic valve intervention, thus TAVI followed by transcatheter intervention for persistent secondary/functional valvular disease is reasonable in this population (Figure 10). Furthermore, the multidisciplinary Heart Team plays a key role to in integrating the plethora of clinical, echocardiographic, haemodynamic, and anatomical data, upon which an optimal management strategy can be planned for each individual.
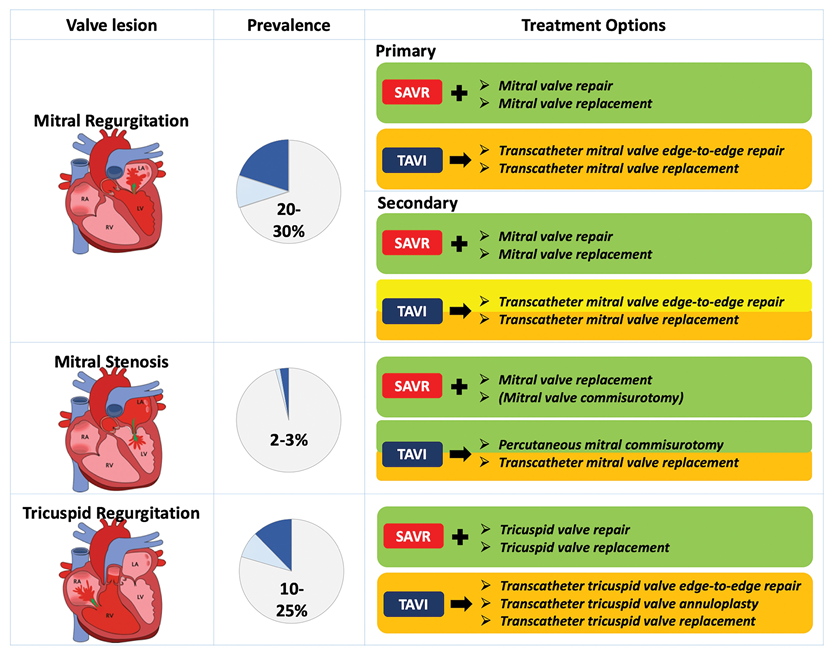
Prevalence of concomitant valve disease in transcatheter aortic valve implantation and potential treatment strategies.
Figures reproduced from Windecker et al. Eur Heart J 2022;43(29):2729-2750.
Anatomical risk stratification based on aortic root anatomy is one of the key considerations in patient selection for TAVI or SAVR. In patients with unfavourable aortic root anatomy, the procedural and device success of TAVI may be diminished, leading to unfavourable clinical outcome. Therefore, Heart Team needs to evaluate typical anatomical considerations before the procedure and determine an individual decision-making (Figure 11).
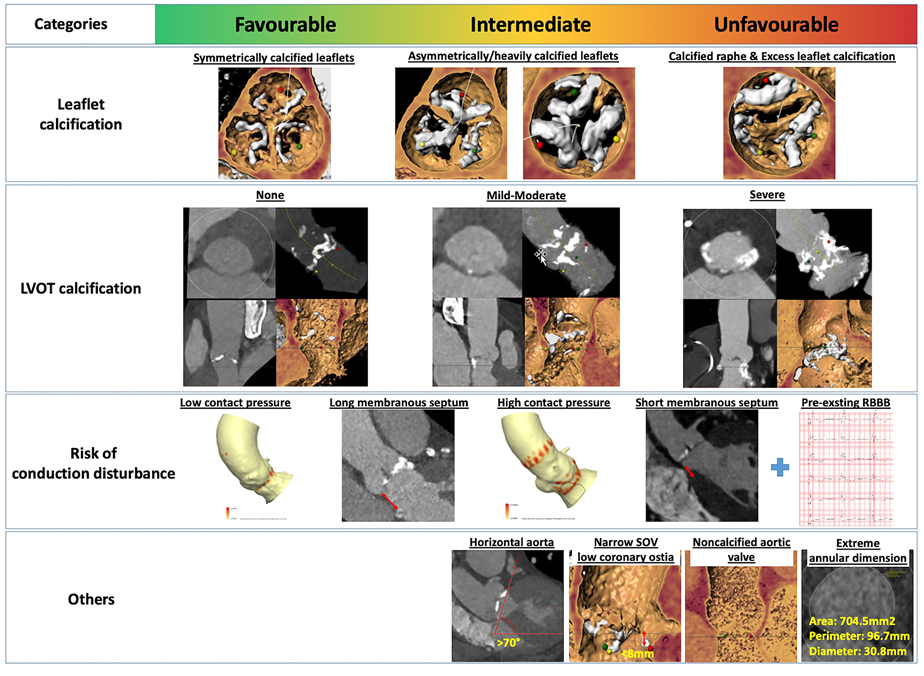
Unfavorable aortic root anatomy for transcatheter aortic valve implantation. Figures reproduced from Windecker et al. Eur Heart J 2022;43(29):2729-2750.
Severely calcified device landing zone is an important anatomical consideration that increases the risk of PVR, annular rupture, coronary obstruction, stroke, and conduction disturbances after TAVI, , , , . Owing to improvements in TAVI technology, the frequency of PVR has declined, , , , while the adverse impact of excess leaflet calcification and severe left ventricular outflow tract (LVOT) calcification remains an important consideration even with newer-generation TAVI devices. In a retrospective analysis of 1,635 patients, moderate or severe LVOT calcification conferred an increased risk of annular rupture when treated with balloon-expandable devices, and a higher incidence of PVR irrespective of valve type or generation. SAVR should be considered in low-risk surgical patients with excessive calcification in the device implantation zone, as the calcified leaflets and calcium extension into the annulus and LVOT can be removed.
The presence of a small aortic root, including small aortic annulus, low-take-off of coronary ostium, and shallow sinus of Valsalva poses a clinical challenge in the management of patients with severe AS. A small aortic annulus may be associated with a high residual prosthetic gradient and prosthesis-patient mismatch resulting in reduced exercise capacity. Although there is no established definition of small aortic annulus (aortic annulus area ≤400 or 430 cm2, aortic annulus perimeter ≤73 mm, etc.), up to 40% of patients with severe AS undergoing SAVR or TAVI have this anatomical feature, , . The optimal treatment strategy in this population has not been determined. TAVI with self-expanding valve has been considered the preferred treatment in patients with small aortic annulus due to improved bioprosthetic haemodynamic outcomes compared with balloon-expandable devices, although it remains unclear whether differences in haemodynamic outcomes translate into clinical benefit, . The randomized SMART trial compared balloon-expandable SAPIEN 3/SAPIEN 3 Ultra with self-expanding Evolut PRO/PRO Plus/FX in patients with small aortic annuli (aortic annulus area ≤430 mm by multi-detector computed tomography [MDCT]). In this unblinded trial, patients allocated to a self-expanding valve had a lower rate of bioprosthetic valve dysfunction and haemodynamic structural valve dysfunction (irrespective of definition of bioprosthetic valve dysfunction [BVD]) and better quality-of-life KCCQ ordinal outcomes compared with patients allocated to a balloon-expandable device at 12 months follow-up. The VIVA trial was a RCT comparing SAVR with TAVI in patients with small aortic annuli defined as aortic annulus mean diameter ≤23 mm or minimal diameter <21.5 mm by MDCT (N = 156). In the VIVA trial, there was no significant difference between TAVI and SAVR with respect to valve haemodynamic results (severe prosthesis-patient mismatch or moderate-severe PVR at 60 days: 5.6% vs. 10.3%, P = 0.30) and clinical outcomes (death, stroke, and cardiac hospitalization).
Another anatomical risk of TAVI related to small aortic root is the risk of coronary obstruction or impaired coronary access in case of low take-off of the coronary ostia in the setting of a shallow sinus of Valsalva, , , . Several procedural techniques have been developed to mitigate the risk of coronary obstruction and to maintain coronary access, which will be discussed in a later section. The decision process in small aortic root anatomy should take into account the expected haemodynamic results, the risk of coronary obstruction and feasibility of coronary protection, the need for future coronary access, and lifetime management with respect to potential risks associated with repeat interventions.
Patients with annulus dimensions beyond the recommended range, extremely large aortic annuli, do not allow for optimal THV sizing. Several reports have summarized the feasibility and safety of TAVI with newer-generation devices using overexpansion in patients with extremely large aortic annuli (annulus area ≥683 mm or perimeter ≥94.2 mm), . In a multicenter study, 124 patients with extremely large aortic annulus had a comparable rate of procedural and in-hospital outcomes after TAVI compared with patients with large aortic annulus (annulus area ≥575 mm or perimeter ≥85 mm) (in-hospital death: 0.8% vs. 2.5%, P = 0.337; in-hospital stroke: 4.0% vs. 1.6%, P = 0.076; device success: 93.1% vs. 94.6%, P = 0.672). It should be noted that these data are currently limited to 1-year follow-up. A bench study has suggested that excessive overexpansion may affect leaflet integrity and hydrodynamic function including regurgitation and durability. A newer-generation balloon-expandable Myval device is available with two additional sizes (30.5 and 32.5 mm), covering larger annulus areas up to 840 mm. Future studies will determine whether optimal sizing TAVI in patients with extremely large annulus results in clinical outcomes comparable to SAVR.
Horizontal aorta, defined as aortic angulation, the angle between the horizontal plane and the plane of the aortic annulus in a coronal projection, greater than 70 degrees, is an important anatomical consideration for TAVI, particularly with some self-expanding devices without steerable delivery systems. Although several studies have evaluated the feasibility of TAVI in patients with higher aortic angulation (e.g., >48 degrees), an inadequate number of patients with horizontal aorta was included, . In a retrospective analysis including 1,214 patients undergoing TAVI with a balloon-expandable SAPIEN 3 or SAPIEN 3 Ultra device, horizontal aorta was observed in 38 patients (3.1%) without apparent impact on procedural and device success and risk of periprocedural complications.
Non-calcified aortic valve morphology has been considered a risk factor for valve embolization or dislocation of THV due to insufficient anchoring of the THV. A single-center observational study of 1,358 patients undergoing TAVI for native severe high-gradient AS reported that patients with less calcified aortic valve, defined as aortic valve leaflet calcium volume ≤163 mm quantified on contrast-enhanced MDCT images with a predefined threshold of 850 HU, had a higher prevalence of autoimmune disease, higher body mass index, female sex, hypertension, and tricuspid valve morphology. Although overall procedural success and clinical outcomes were similar in patients with less calcified AS, these results suggest a distinct pathophysiological and clinical entity. Several studies have suggested that the detection of fibrotic tissue provides a more accurate anatomical risk assessment in patients with AS, particularly in those in whom leaflet fibrosis rather than calcification is a major contributor to valve obstruction, , . A group from the nationwide POL-TAVI registry proposed a semi-automated method to identify and quantify aortic valve tissue components as calcific and non-calcific and reported that their method correlated well with histopathological quantification, . In addition, they reported that non-calcific aortic valve tissue volume was associated with a five-fold increased risk of MACE at 30 days after TAVI.
The established evidence for TAVI, particularly in the recent low-risk trials, is predominantly based on transfemoral TAVI, and current guidelines assign a Class I recommendation for transfemoral TAVI, . In contrast, the available evidence for non-transfemoral TAVI is limited to observational studies and subgroup analyses in early randomized trials, , , , . Although several studies have suggested that the benefits of non-transfemoral TAVI are similar to those of transfemoral TAVI, the transfemoral approach is generally the least invasive and standard strategy to perform TAVI. Indeed, in the transthoracic TAVI cohort of the PARTNER 2A trial, TAVI was inferior to SAVR in terms of death or disabling stroke at 5 years (59.3% vs. 48.3%, P = 0.03). Therefore, the evaluation of iliofemoral access anatomy is important in the choice between SAVR and TAVI and between transfemoral and non-transfemoral access (Figure 12). Recently, a simplified scoring system based on preprocedural MDCT, the Hostile score, has been proposed to quantify the extent of atherosclerosis and assess the risk of complications in patients with peripheral artery disease undergoing TAVR. The scoring system was validated in a large single-center TAVI registry of 2,023 patients undergoing transfemoral TAVI with a contemporary device and demonstrated that the Hostile score was an independent predictor of non-puncture site vascular complications, whereas its performance in predicting puncture site vascular complications was modest.
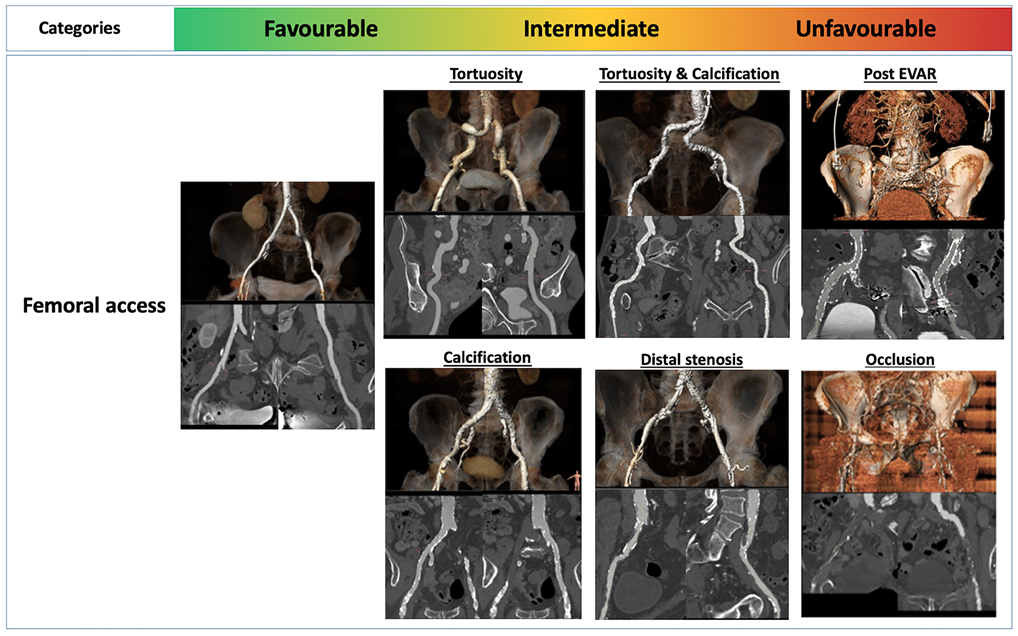
Unfavorable iliofemoral arterial anatomy for transcatheter aortic valve implantation. Figures reproduced from Windecker et al. Eur Heart J 2022;43(29):2729-2750.
Coronary access after TAVI can be challenging if the native leaflet is longer than the coronary height, particularly in a narrow sinus of Valsalva. If the stent frame of the THV extends above the take-off of the coronary arteries, misalignment of the stent frame with the native commissures (or the take-off of the coronary arteries in case of eccentric coronary take-off) further complicates coronary access (Figure 13), . The difficulty is highlighted after valve-in-valve TAVI (TAV-in-SAV or TAV-in-TAV) due to a Neoskirt potentially extending above the coronary ostia or in case of TAV-in-TAV with unfavourable overlap of stent frame struts (Figure 14), , . The rate of unsuccessful selective coronary cannulation is particularly noteworthy with self-expanding THV with a tall stent frame extending beyond the take-off of the coronary arteries, , . Implications of challenging coronary access are accentuated in patients presenting with acute myocardial infarction, although the incidence of ST-segment elevation myocardial infarction after TAVI is low (<1%). There is limited data on repeat coronary intervention after AVR but known CAD increases the risk of future interventions, . The integrated assessment of future coronary access after AVR, including AVR modality and type of THV, should be included in the decision-making process of treatment strategies in patients with severe AS.
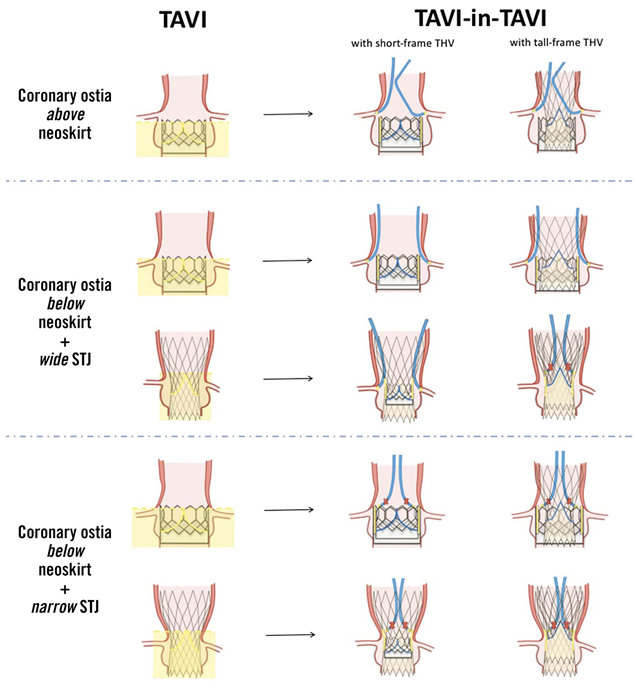
Coronary access after transcatheter aortic valve implantation. Figures reproduced from Tarantini et al. EuroIntervention 2023;19(1):37-52.
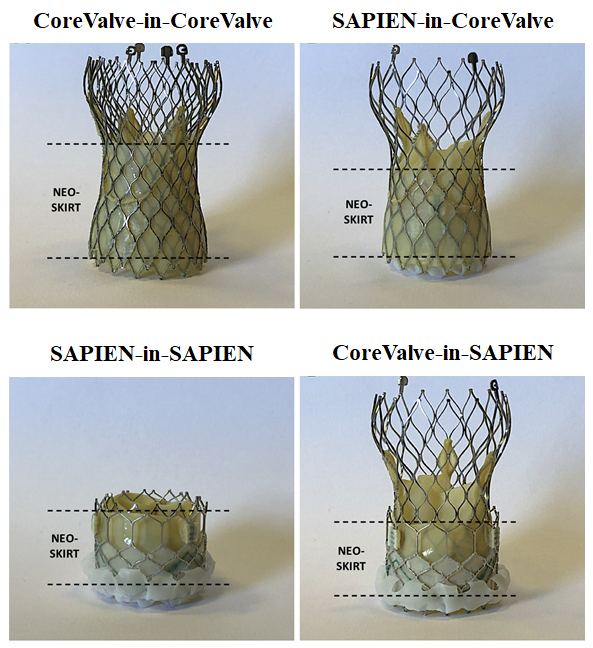
Redo-transcatheter aortic valve implantation: different THV–in–THV combinations.
Figures reproduced and modified from De Backer et al. JACC Cardiovasc Interv 2020;13(21):2528-2538.
The value of the Multidisciplinary Heart Team has become increasingly apparent, and decisions concerning treatment and intervention should be made by a collaborative Heart Team with expertise in valvular heart disease. Key members of the Multidisciplinary Heart Team include clinical and interventional cardiologists, cardiac surgeons, imaging specialists with expertise in interventional imaging, cardiovascular anaesthesiologists, dedicated cardiovascular nurses, and other specialists if necessary (e.g. heart failure specialists or electrophysiologists). Current guidelines recommend that aortic valve interventions must be performed in Heart Valve Centres that declare their local expertise and outcomes data, have active interventional cardiology and cardiac surgical programmes on site, and a structured collaborative Heart Team approach (Figure 15), , .
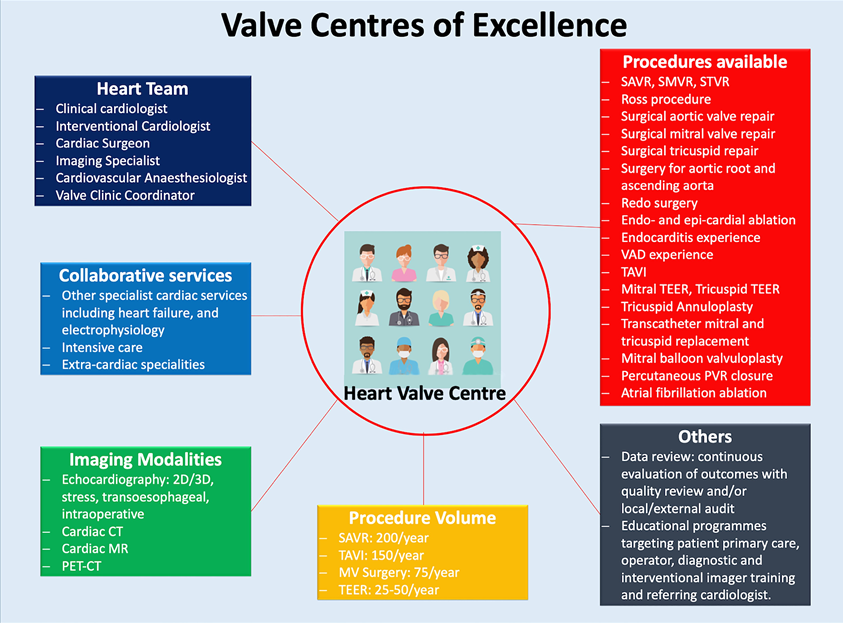
Comprehensive heart valve centre.
Figures reproduced from Windecker et al. Eur Heart J 2022;43(29):2729-2750.
Pre-procedural planning is of critical importance when considering TAVI. Operators should integrate clinical and imaging data obtained by MDCT, echocardiography, and cardiac catheterization to determine the best procedural strategy. Because various TAVI devices are available (Figure 8), operators need to be familiar with the strengths and limitations of each device, . Device sizing should be carefully determined based on the manufacturers’ sizing chart and annulus dimensions derived by MDCT. In patients with unfavourable iliofemoral anatomy, the decision to perform concomitant peripheral interventions (balloon angioplasty, intravascular lithotripsy, use of parallel stiff wire, or surgical cut-down) and the selection of alternative access sites should be determined by patients’ anatomic features and comorbidities as well as local experience. Preventative strategies and management of potential complications should also be discussed before the procedure. If patients are deemed at high-risk of coronary obstruction, operators need to consider coronary protection (more detailed description will be provided in a later section) and emergency coronary revascularization. For patients deemed at high risk of intraprocedural cerebrovascular events, such as excessive aortic valve leaflet calcification, cerebral embolic protection devices may be considered to capture or deflect emboli to the brain (Figure 16), , . It should be noted, however, that there is no robust evidence that the use of cerebral embolic protection devices reduces the incidence of cerebrovascular events, , .
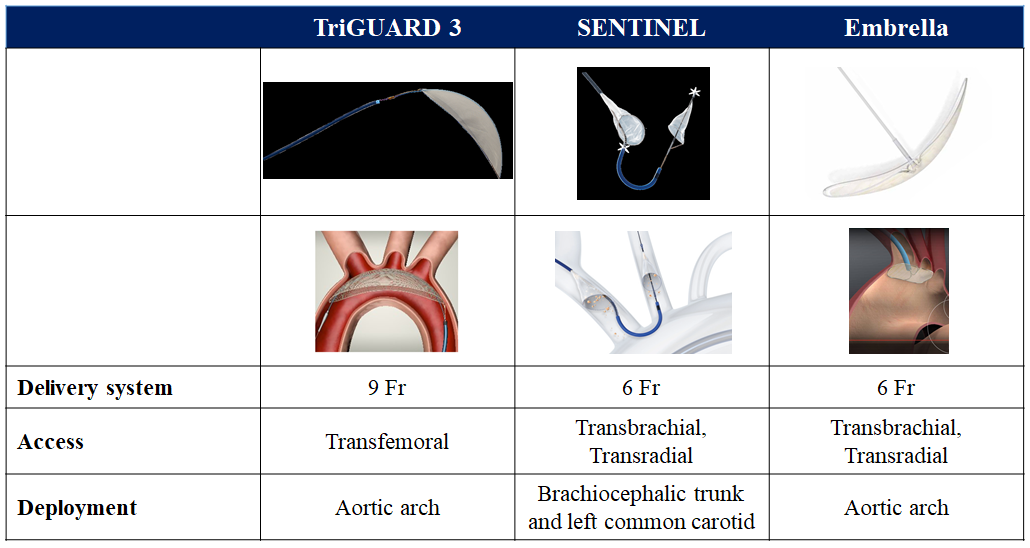
Cerebral embolic protection devices.
The TAVI procedure can be performed in a hybrid operating room or cardiac catheterization laboratory under general anaesthesia or local anaesthesia with or without conscious sedation. In the randomized SOLVE-TAVI trial comparing local anaesthesia with conscious sedation versus general anaesthesia in patients undergoing TAVI, local anaesthesia with conscious sedation resulted in lower rates of catecholamine use (62.8% vs. 97.3%, P <0.001) and the primary composite endpoint of all-cause death, stroke, myocardial infarction, infection requiring antibiotic treatment, and acute kidney injury at 30 days was similar between groups (27.2% vs. 26.4%, P for equivalence = 0.015). As the learning curve of the TAVI procedure continues to improve in addition to the expansion into lower risk patient populations, TAVI under local anaesthesia with or without conscious sedation has become the standard strategy in most institutions. In the STS/ACC TVT registry, the use of conscious sedation has increased from 33% in 2016 to 64% in early 2019, with a wide variability in the use of general anaesthesia, with a few centres using only general anaesthesia. The shift represents the elimination of routinely used transesophageal echocardiography, orotracheal intubation, and other invasive monitoring techniques. Currently general anaesthesia is typically reserved for non-femoral access TAVI or anticipated complex procedures.
Among patients without indication for long-term oral anticoagulation (OAC), pre-procedural loading with low-dose aspirin is adopted and procedural anticoagulation is achieved with administration of unfractionated heparin maintaining an activated clotting time of 250-300 seconds. Bivalirudin or Argatroban can be used alternatively in patients with a history of heparin-induced thrombocytopenia. In a randomized trial comparing bivalirudin and heparin as the procedural anticoagulant agent in patients undergoing transfemoral TAVI, bivalirudin did not meet superiority criteria for major bleeding at 48 hours (6.9% vs. 9.0%, P = 0.27), but was found non-inferior in terms of net adverse cardiovascular events including all-cause death, myocardial infarction, or stroke at 30 days (14.4% vs. 16.1%, Pnon-inferiority <0.01).
Haemostasis may not be adequately achieved under the anticoagulant effect of heparin. Heparin reversal by the administration of protamine is routinely used in cardiac surgery to reduce bleeding complications. Accordingly, protamine administration at the time of sheath removal and closure of the arteriotomy site can be considered in patients undergoing transfemoral TAVI. Available data is limited to one RCT and a few observational studies, and potential risks of protamine including allergic reactions, hypotension, bronchospasm, and skin reactions have been reported. The ACE-PROTAVI trial was an investigator-initiated, placebo-controlled randomized clinical investigating of the efficacy and safety of routine protamine administration after transfemoral TAVI. In this trial, 410 patients were randomly assigned to either the protamine or placebo groups. Compared with patients receiving placebo, patients receiving up-front protamine administration had a higher rate of haemostasis success (97.9% vs. 91.6%, P = 0.006), a shorter median time to haemostasis (181 seconds vs. 279 seconds, P = 0.002), and a reduced risk of the composite of all-cause death, major and minor bleeding complications, or major and minor vascular complications at 30 days (5.2% vs. 12.8%, P = 0.01), predominantly driven by the difference in the prevalence of minor vascular complications (2.1% vs. 8.4%, P = 0.01).
Among patients with indication for long-term OAC, the decision to continue or discontinue OAC during cardiovascular interventions requires balancing the risk between thromboembolic and bleeding complications. In certain cardiac interventions, including catheter ablation for atrial fibrillation, pacemaker implantation, and PCI, continuation of OAC is associated with lower rates of bleeding and similar or even lower rates of embolic complications, , . Recent observational data suggest that there is favourable association between continued OAC and clinical outcomes in TAVI population, . The POPular PAUSE TAVI randomized control trial evaluated the safety and efficacy of periprocedural continuation versus interruption of OAC during TAVI. In this trial, 869 patients with an indication for long-term OAC use undergoing transfemoral or transsubclavian TAVI were randomly assigned to a continued or interrupted OAC strategy. In the interrupted strategy group, OAC regimens were interrupted 48-120 hours before TAVI, depending on the type of OAC, and renal function and heparin bridging was not initiated. At 30 days, the primary composite endpoint of cardiovascular mortality, all stroke, myocardial infarction, major vascular complications, or major bleeding after TAVI was 16.5% in the continued strategy group and 14.8% in the interrupted strategy group, respectively, and the continued strategy did not meet the non-inferiority criteria for the primary endpoint (Pnon-inferiority = 0.18). Although the rate of thromboembolic events was similar between groups, more bleeding complications (all and major bleeding) occurred in the continued strategy group. These results suggest that interruption of OAC is reasonable in patients undergoing TAVI as compared to other cardiac procedures due to their older age, advanced frailty, and comorbidities.
Patients with prosthetic valves are at increased risk for infective endocarditis. Considering the worse outcomes of patients with prosthetic valve endocarditis as compared to patients with native valve endocarditis, clinical guidelines for the management of infective endocarditis recommend antibiotic prophylaxis (Class I recommendation). During transcatheter valve procedures, staphylococcal and enterococcal species predominate are common, with enterococcal infections being more prevalent periprocedurally, potentially related to the flora colonizing the groin when transfemoral access is used. This underscores the focus on procedural infection prevention, including aseptic measures during the insertion and manipulation of catheters using surgical standards, as well as prophylaxis. With regards to antibiotic prophylaxis, the guidelines recommend antibiotic prophylaxis to cover for Enterococcus spp:
Vascular closure devices.
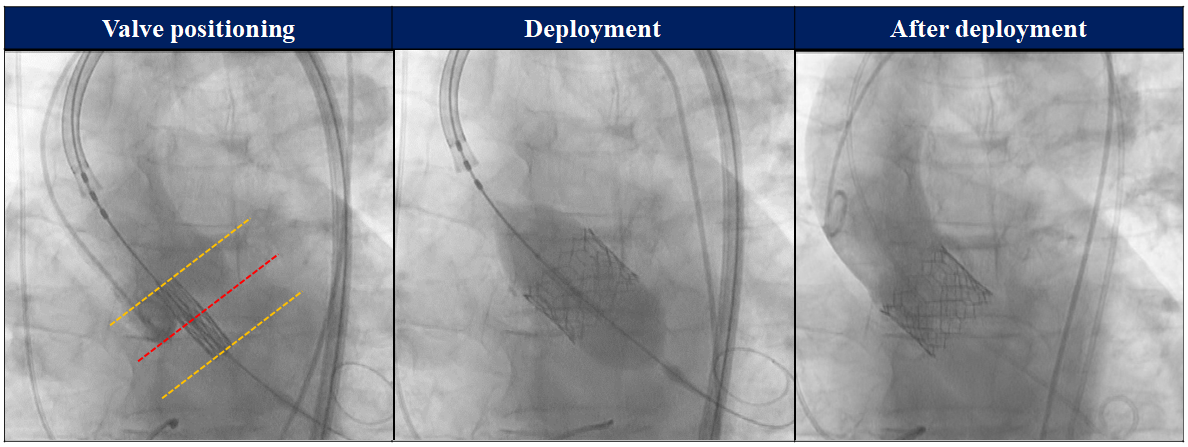
Transcatheter heart valve deployment: SAPIEN system.
Valve alignment on the delivery catheter balloon is performed and confirmed in a straight section of the descending aorta. Once the THV has passed the aortic valve, the balloon lock is disengaged and the tip of the Flex Catheter is retracted to the centre of the three markers, followed by re-engaging the balloon lock. The Flex wheel is used in combination with gentle manipulations of the guidewire and handle to adjust the coaxiality of the THV within the annulus and the fine adjustment wheel serves to fine-tune the position of the THV across the valve. Before deployment, it is ensured that the THV is accurately positioned between the alignment markers. Under rapid ventricular pacing (to decrease systolic pressure to 50 mmHg or less), deployment is performed with slow, controlled inflation until the complete predetermined volume within the indeflator has been administered and maintained for 3 seconds. When the balloon catheter is completely deflated, pacing is discontinued and the delivery system removed.
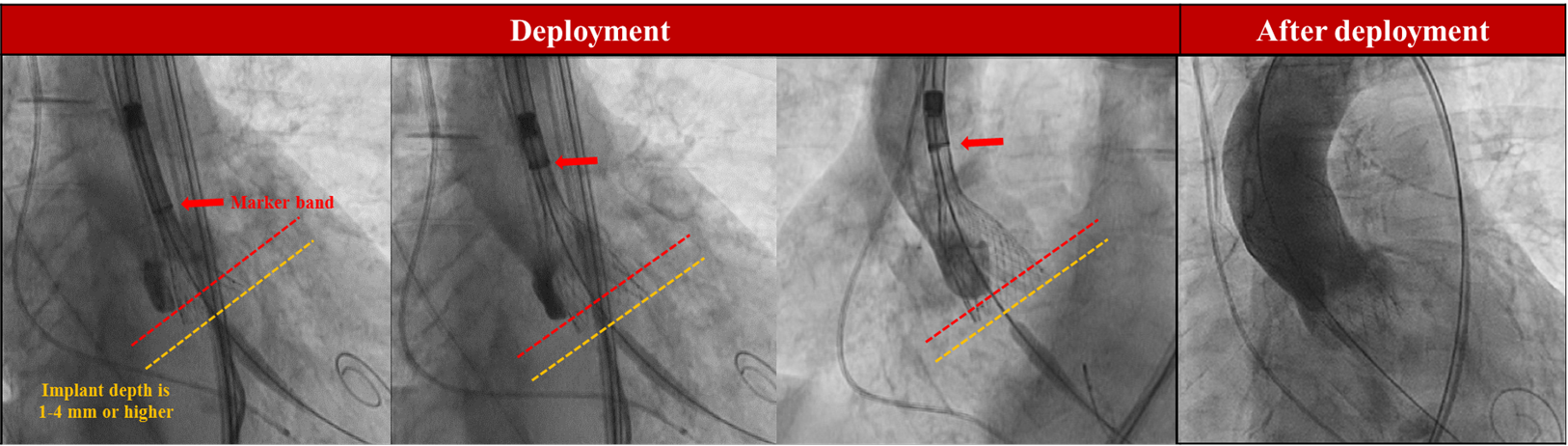
Transcatheter heart valve deployment: Evolut system.
Once the THV is advanced into appropriate implant position (targeting an implant depth of 1-4 mm), deployment (unsheathing) is performed by rotating the deployment knob. Controlled pacing (90 to 120 bpm) is frequently used during the deployment to stabilize the valve during flaring. Periodic aortic root injections guide proper positioning during deployment, which is generally initiated in a cusp-overlap view. Once blood pressure drops due to temporary occlusion of the aortic valve by the expanding THV, the operator continues to turn the deployment wheel until blood pressure recovers. A tactile indicator provides feedback to indicate that the capsule is nearing the “point of no recapture” (at approximately 2/3 deployed). At this point, the imaging projection should be adjusted to remove the parallax in the valve inflow to determine valve position using aortic root angiography and deployment height at the left coronary sinus should be verified in the 3-cusp coplanar view. If the operators are satisfied with the valve position and performance (aiming for no more than 1-4 mm extension into the LVOT), wire-tension is relieved and the THV is slowly fully released. The detachment of frame paddles must be confirmed under fluoroscopy, and the nose cone centered before the delivery system is withdrawn.
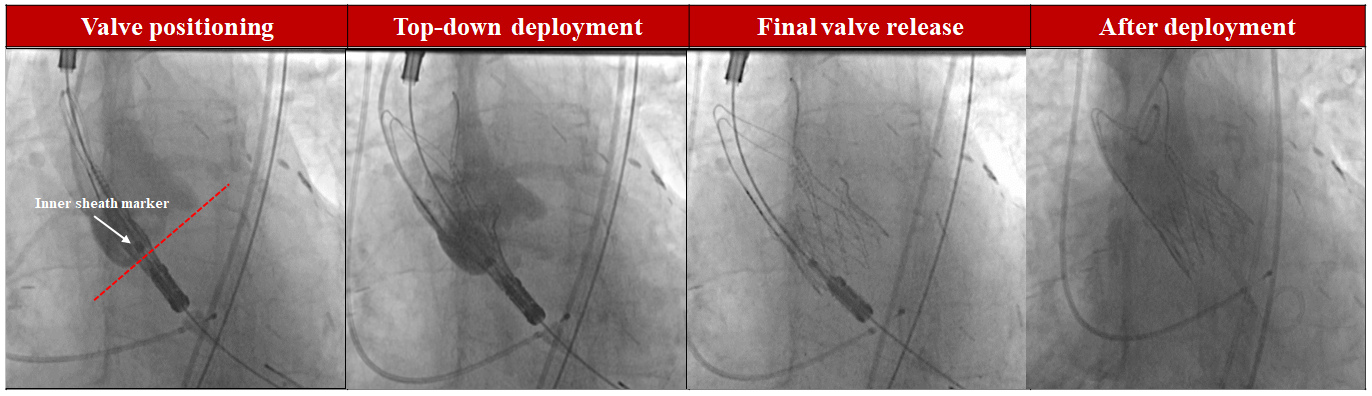
Transcatheter heart valve deployment: Acurate system
In contrast to other self-expanding THV systems, the ACURATE series THV are released from the top downwards with a 2-step procedure. Due to the moderate radial force of the ACURATE series THV, effective balloon predilatation is recommended to facilitate device expansion. Once the THV has passed the aortic valve, ensure that the THV is correctly positioned as indicated by the radiopaque intersection line (marker band), which is located in the mid-portion of the stent body using the annular plane. In addition, the upper crown should be located right above the tips of the native leaflets. After the proper initial position is achieved, the first step can be initiated by turning knob 1 counter-clockwise until full stop, releasing the upper crown and stabilization arches. This step should be performed in a controlled fashion to detect any inappropriate movement of the device. Aortic root angiography should be used to verify the position of the THV. Even after completing step 1, it is still possible to adjust the position. If the operator is satisfied with the position, knob 2 will be turned counter-clockwise to release the lower crown for full deployment of the THV. The detachment of the frame paddles must be confirmed under fluoroscopy, and the nose cone centred before the delivery system is withdrawn.
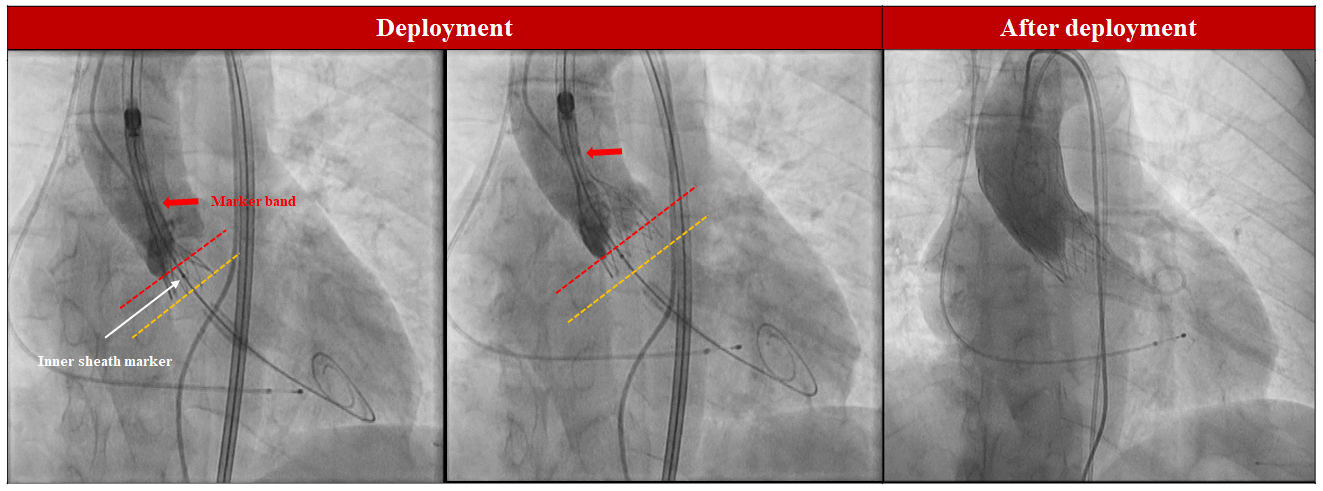
Transcatheter heart valve deployment: Navitor system.
Once the THV is advanced across the aortic valve, the radiopaque marker on the FlexNav delivery catheter is aligned with the annular plane. Deployment is then initiated by turning the deployment wheel, typically under controlled pacing (90 to 120 bpm). A clicking sound is heard when the delivery system reaches the partial deployment lock. The deployment mechanism will not re-engage until the deployment lock button is activated. At this point, the imaging projection should be adjusted to eliminate the parallax in the valve inflow and confirm the valve positioning using aortic root angiography. Once proper positioning is confirmed, wire-tension is relieved and the valve completely deployed by pressing the deployment lock button and rotating the deployment wheel in the direction of the arrow on the handle until the valve capsule is fully retracted. Fluoroscopy should be used to confirm release of the retainer tabs before withdrawing the system.
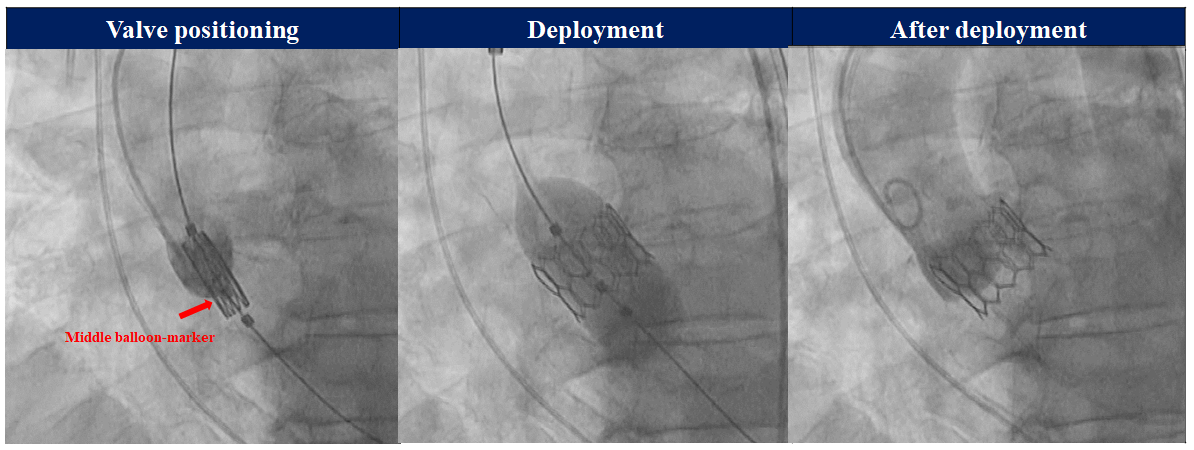
Transcatheter heart valve deployment: Myval system.
The balloon-expandable Myval series THV is crimped directly onto the balloon catheter delivery system. The correct valve alignment on the balloon needs to be confirmed in a straight section of the descending aorta. Under fluoroscopy, dark and light bands can be identified, which allow the operator to position the THV at its designated landing zone, the middle or second dark band on the balloon catheter from the ventricular end (70-80% in the aorta: 20-30% in the ventricle). In case of a challenging anatomy where the THV cannot cross the stenotic valve, the undeployed Myval can be fully retrieved through the iliofemoral sheath. Once the THV has crossed the aortic valve, deployment is performed under rapid pacing, with slow, controlled inflation until the full predetermined volume is administered and maintained for 3 seconds. When the balloon catheter is completely deflated, turn off the pacemaker and remove the delivery system.
Conventionally, the implantation depth is aimed at an aortic frame to LVOT ratio of 80:20 for balloon-expandable THV and 1-4 mm below the aortic annulus for self-expanding THV Since implantation depth correlates with conduction disturbances, a high deployment technique has been proposed to achieve a lower risk of conduction system impairment after TAVI. Although several modifications are used, the key features of the implantation technique are consistent: for the balloon-expandable SAPIEN THV, the THV is positioned by aligning the radiolucent line that is located on the superior aspect of the lowest set of stent struts of the crimped valve, at the base of the non-coronary cusp; for the self-expanding CoreValve/Evolut THV, the THV is implanted using the cusp-overlap view with a target implantation depth of 0 to 3 mm, , , , , . In a single center observational study of 160 and 258 patients treated with Evolut R/PRO/PRO Plus and SAPIEN 3 THV, respectively, high implantation techniques had a lower incidence of new conduction disturbances (new permanent pacemaker implantation and new LBBB) at 30 days after TAVI compared with conventional technique for both THV (pacemaker: 0.0% vs. 10.8%, P = 0.02; LBBB: 4.2% vs. 11.3%, P = 0.22 in the Evolut THV and pacemaker: 2.0% vs. 2.2%, P = 1.0; LBBB: 1.1% vs. 7.0%, P = 0.047 in the SAPIEN 3 THV). However, post-TAVI MDCT showed that risk of sinus sequestration in redo-TAVI was significantly higher in the high implantation group compared with the conventional implantation group (Evolut THV: 64.0% vs. 41.8%; P = 0.009; SAPIEN 3 THV: 17.6% vs. 5.3%; P = 0.002).
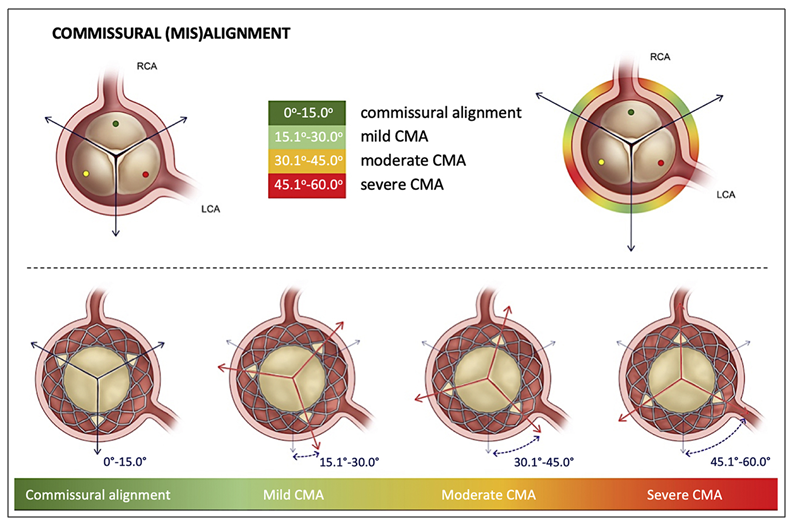
Proposed standardized definition of commisural alignment from the ALIGN-TAVR consortium. Figures reproduced from Tang et al. JACC Cardiovasc Interv 2022;15(15):1497-1518.
In contrast to SAVR, in which the commissural posts are aligned with the native aortic valve commissures distant from the coronary ostia, TAVI may result in the inadvertent placement of THV commissural posts facing a coronary orifice, which renders coronary access challenging. In the multicentre RE-ACCESS and ALIGN-TAVR registries, commissural misalignment was an independent predictor of unsuccessful coronary cannulation after TAVI, . In addition, commissural misalignment is associated with changes in the fluid flow patterns, increased leaflet stress, and creating nonphysiological vorticity, leading to faster bioprosthetic degeneration. Therefore, achieving proper commissural alignment is important in lifetime management considerations of patients with severe AS. The ALIGN-TAVR Consortium has proposed preprocedural planning and implantation techniques to achieve commissural alignment. Based on the CT-derived “3-cusp” and “cusp-overlap” views are used during the deployment. For the CoreValve/Evolut THV, a C-paddle locates at 1 of the commissures and is mounted 90º clockwise from the fluoroscopic “Hat” marker. By inserting the delivery catheter with the flush port at 3 o’clock (away from the operator), the “Hat” marker can be better oriented to the centre front in the cusp overlap view during valve implantation, thus improving commissural alignment. With successful commissural alignment, the C-paddle should end up at the inner curve of the ascending aorta in the cusp overlap view. For the ACURATE THV, the commissures can be identified on fluoroscopy by the presence of 3 commissural posts at the base of the stabilizing arches and 3 “free cells” at the level of the upper crown. By inserting the delivery system with the flush port facing 6 o’clock, fine-tuning adjustment (clockwise or counterclockwise rotation) is applied to optimize alignment. The RE-ACCESS 2 study evaluated the rate of unsuccessful coronary access after TAVI in 127 patients who undergoing TAVI with a self-expanding Evolut or Acurate THV using commissural alignment technique. In this study, only 7 patients (5.5%) had unsuccessful coronary cannulation after TAVI and commissural misalignment affected coronary cannulation after TAVR mostly in Evolut THV. These results demonstrate the utility of the commissural alignment technique. However, it should be noted that a non-negligible proportion of patients have severely eccentric origins of the coronary arteries, particularly in the right coronary artery (>10% of cases), which may compromise commissural alignment even with commissural alignment techniques. Furthermore, there is currently no established straightforward technique to obtain commissural alignment for other types of THV.
In patients with borderline aortic annulus areas that fall in between two valve sizes, the operator is faced with the decision to oversize or undersize the valve prosthesis. In case of balloon-expandable devices, under- or over-filling the delivery catheter balloon volume by a few milliliters with respect to manufacturer’s specifications is frequently used in current TAVI practice. Ex vivo bench studies have reported that over-expansion may be associated with reduced leaflet mobility and variable degrees of leaflet tear, and that under-expansion may lead to a pinwheeling phenomenon, twisting of the free edges of the leaflets, resulting from excessive leaflet redundancy. A prospective study including 565 patients with post-TAVI MDCT reported that non-uniform expansion and under-expansion of TAVI prostheses may result in frame deformation, asymmetric leaflets, and smaller neosinus volume, which has been associated with hypoattenuated leaflet thickening (HALT). In a single-centre retrospective study, patients who received a suboptimally sized THV experienced an increased risk of all-cause and cardiovascular death at 1 year compared with those who received an optimally sized THV (adjusted HR 1.42, 95% CI 1.0-2.0, P = 0.047 and adjusted HR 1.59, 95% CI 1.06-2.39, P = 0.026, respectively). A newer-generation balloon-expandable device (Myval) introduces a sizing strategy with 1.5 mm diameter increments between nominal device sizes, allowing for a more nuanced sizing strategy to match the aortic annulus compared to conventional devices with fixed 3.0 mm diameter increments. However, in the COMPARE TAVI 1 trial, Myval THV had a higher incidence of moderate or greater aortic regurgitation compared with SAPIEN THV.
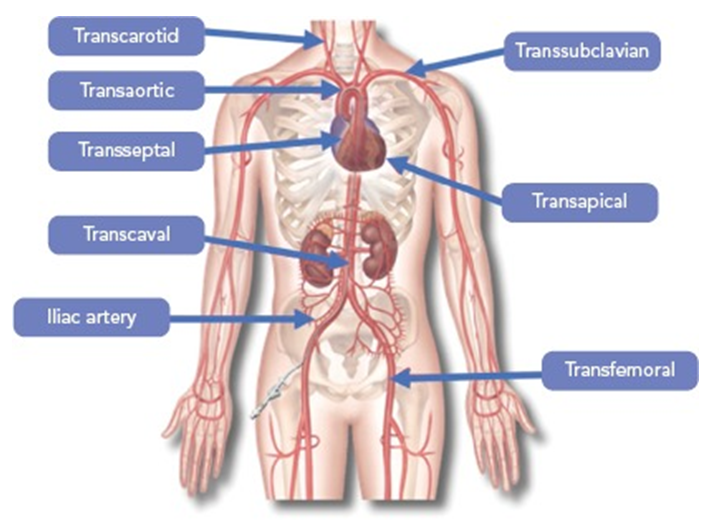
Access sites for transcatheter aortic valve implantation. Figures reproduced from Overtchouk and Modine. Interv Cardiol 2018;13:145-150.
Transapical TAVI is performed via a left anterolateral intercostal incision followed by needle puncture of the apex through a pledgeted purse-string suture. A dedicated sheath is then placed and the THV is deployed in a similar manner to the transfemoral approach. This approach is typically used for the Edwards SAPIEN platform. Of note, this access site is associated with delayed recovery due to its invasiveness. In the PARTNER IA trial, the risk of all-cause mortality at 5 years was 79% in the transapical TAVI group versus 60% in the SAVR group (P = 0.067). Similarly, in the PARTNER II trial, transthoracic TAVI (including both transapical and transaortic) was associated with a higher rate of death or disabling stroke at 5 years compared with SAVR.
Transaortic access is performed through the ascending aorta via a right anterior mini-thoracotomy in the second intercostal space. The aorta is inspected to avoid areas of calcification. A purse-string suture is used to obtain a needle puncture and access with a dedicated sheath. The puncture is made with a minimum of 6 cm above the aortic annulus for the CoreValve platform and 8 cm for the SAPIEN platform. The valve is then deployed in the same manner as via the transfemoral approach.
Transsubclavian/axillary approaches are the most commonly used alternative TAVI access sites in 2018 and 2019 in the STS/ACC TVT registry. Transsubclavian access is typically performed via surgical cut-down, while trans-axillary can be performed percutaneously with pre-closure sutures most frequently into the proximal third of the axillary artery. There is no dedicated sheath available. A left-sided approach is preferred as it provides a more favourable alignment of the THV with the native valve. The presence of the left internal mammary artery graft in patients with coronary artery bypass surgery is considered a relative contraindication in the absence of native coronary artery flow to the left anterior descending artery or other territory that is supplied by the graft. In a retrospective analysis of the STS/ACC TVT registry, transsubclavian/axillary approach was used in 1,249 (34.4%) of 3,628 patients undergoing alternative access. After propensity-matching, trans-subclavian/axillary approach had lower 30-day mortality (5.3% vs. 8.4%, P<0.01), shorter lengths of intensive care unit and hospital stay, but a higher stroke rate (6.3% vs. 3.1%, P <0.05) compared with transthoracic approach.
Transcarotid access is performed percutaneously under local anaesthesia or via surgical cut-down and requires cerebral oximetry monitoring. Satisfactory vessel size and vessel quality are critical determinants for efficacy and safety as is an anatomically complete Circle of Willis. In a retrospective analysis of a multicentre registry including 329 alternative access TAVI patients (2012-2017), transcarotid TAVI was associated with less new-onset atrial fibrillation (3.2% vs. 19.0%, P = 0.002), acute kidney injury (0% vs. 12.1%, P = 0.002), and shorter median length of hospital stay (6 days vs. 8 days, P <0.001) compared with transthoracic TAVI after propensity-matching.
Transcaval access requires a dedicated pre-TAVI planning derived from the preprocedural MDCT. A calcium-free target on the right aortic wall that allows safe passage of the TAVI sheath from the inferior vena cava to the abdominal aorta needs to be identified. The trajectory of the sheath must be free of interposed obstacles (bowel), and the area of aortic entry should avoid important arterial branches, which allows for provisional covered stent bailout if needed. In brief, the femoral vein is accessed and a coronary guidewire (0.014 inch) connected to electrocautery is used to cross from the inferior vena cava into the abdominal aorta where it can be snared and advanced towards the aortic arch. The coronary guidewire is then exchanged for a stiff guidewire (0.035 inch). The TAVI sheath can be introduced via the stiff wire and the procedure can carry on as in the case of transfemoral TAVI. Once the THV has been deployed, a nitinol cardiac occluder (generally an Amplatzer Duct Occluder I) is used to seal the arteriotomy in the abdominal aorta. In a multicentre European study, transcaval TAVI was successful in 49 out of 50 patients suggesting it to be feasible and safe for high-risk AS patients who are not suitable for transfemoral or transsubclavian/axillary access. These findings are supported by a recent meta-analysis from 8 studies with 467 patients undergoing transcaval TAVI.
Owing to improvements in TAVI technology and techniques, the rate of procedural complications has decreased over recent years, , . Notwithstanding, TAVI-related complications can occur in a non-negligible proportion of patients and may impact patient prognosis (Figure 26).
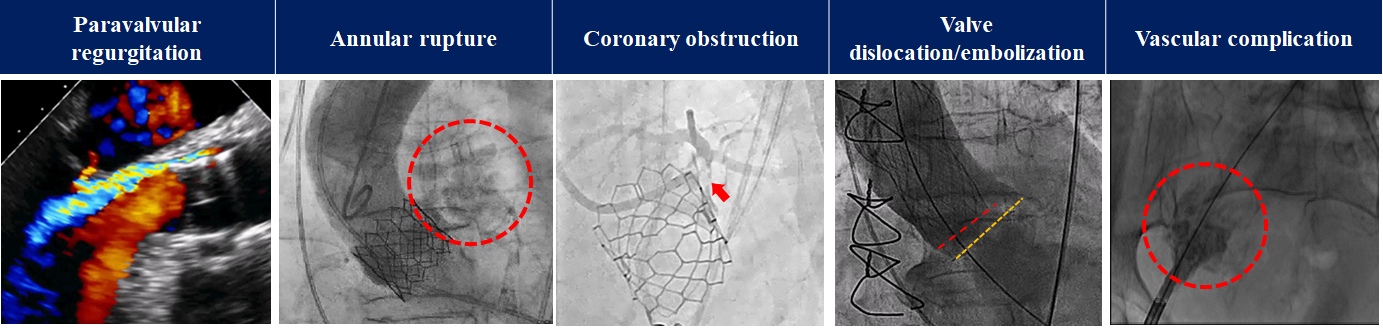
Procedural Complications.
PVR occurs with incorrectly positioned THV implantation (too high or too low), undersized THV, or the presence of morphologic features that prevent ideal device apposition, such as asymmetric, protruding device landing zone calcification. PVR is typically assessed by post-procedural aortic root angiography and/or transthoracic or transesophageal echocardiography. Since significant PVR is associated with an increased risk of adverse events after AVR, , , , additional procedures should be considered if significant PVR is observed and the expected mechanism is addressable. If the leak is due to underexpansion of the THV, post-dilation should be performed to obtain optimal expansion. In case of THV malposition, a valve-in-valve procedure may be considered. In a multicenter study of 201 patients who underwent transcatheter intervention for the treatment of moderate or greater PVR after TAVI, PVR was successfully reduced to ≤mild in 82.6% of patients, and patients in whom ≤mild PVR was achieved had lower 1-year mortality than those with residual moderate or greater PVR (8.0% vs. 21.4%, P = 0.007). Although the incidence of significant PVR has decreased, the incidence of mild PVR is still high compared with SAVR, , , , , , , , , . In a single-center TAVI registry of 1,128 patients (568 with no/trace PVR and 560 with mild PVR), patients with mild PVR had a higher risk of mortality than those with none/trace PVR at 5 years (54.6% vs. 43.8%; adjusted HR: 1.26, 95% CI: 1.06-1.50). When applying the 5-class grading scheme, only mild-to-moderate PVR was associated with an increased risk of mortality at five years (mild PVR: adjusted HR: 1.19, 95% CI: 0.99-1.43, mild-to-moderate PVR: adjusted HR: 1.56, 95% CI: 1.20-2.02).
Aortic annular rupture is a rare (<1%) but life-threatening complication of TAVI requiring prompt rescue for haemodynamic stabilization. Annular rupture is more common with balloon-expandable THV or following aggressive pre/post balloon dilation of any valve type in the setting of a severely calcified valve with extension into the aortic root and LVOT, . In general, annular rupture is detected by aortic root angiography and/or echocardiography with pericardial effusion. However, in some cases such as infra-annular injury, transesophageal echocardiography may be required for its detection. Furthermore, the clinical manifestations may vary depending on the location and extent of the injury. When rapid haemodynamic collapse occurs and is unexplained, the presence of annular rupture should be considered. Although conservative management (isolated pericardial drainage and/or optimization of the coagulation status) is selected in certain cases, surgical bailout is the default treatment strategy in patients with haemodynamic collapse. However, patients with annular rupture may not be stabilized to undergo surgical bailout in view of rapid clinical deterioration. Recently, rescue TAV-in-TAV has been suggested as rescue strategy to seal the site of annular rupture by increasing apposition of the first THV and extending the skirt-covered frame height by implantation of the second THV. This strategy could prevent further haemodynamic deterioration and may obviate the need for emergent sternotomy in suitable cases.
The mechanisms of TAVI-related coronary obstruction fall into two categories. Direct coronary obstruction occurs when the THV displaces the degenerated native or bioprosthetic valve leaflets toward the ostia of the coronary arteries; this typically occurs in patients with low coronary take-off combined with a shallow configuration of the sinus of Valsalva. Conversely, indirect coronary obstruction may occur in patients with a low and narrow sinotubular junction through the mechanism of sinus sequestration (Figure 27). This is a rare but a life-threatening complication of TAVI, , . In the nationwide Spanish TAVI registry (13,675 patients between 2009-2021), the incidence of coronary obstruction was 0.68% for native TAVI and 5.7% for valve-in-valve TAVI. Among patients who developed coronary obstruction, rescue coronary revascularization was performed in 90.4% (PCI 86.9% and CABG 3.5%), and 22% of rescue PCI procedures were unsuccessful resulting in higher in-hospital mortality (patients without coronary obstruction: 4.1%; patients with coronary obstruction receiving successful revascularization: 20.7%; and patients with coronary obstruction receiving unsuccessful revascularization: 78.8%). Of note, coronary obstruction can develop with delay after the index TAVI procedure with a similar clinical impact as acute obstruction. Therefore, the risk of coronary obstruction must be taken into consideration during pre-procedural planning. Several anatomical risk factors for coronary obstruction have been identified, , . A preprocedural MDCT-based risk prediction model for direct coronary obstruction during native TAVI has been proposed using the data from the CO-TAVR and COBRA registries. This model includes cusp height > coronary artery height and virtual transcatheter valve-to-coronary-ostium (VTC) distance 4 mm or culprit leaflet calcium volume >600 mm and shows a high predictive performance (area under the curve [AUC] of 0.93, a sensitivity of 0.93, and a specificity of 0.84 for the left coronary artery and with an AUC of 0.94, a sensitivity of 0.92, and a specificity of 0.96 for right coronary artery). Similarly, the VIVID registry documented that a shorter VTC distance predicted coronary obstruction and proposed a cutoff value of 4 mm in patients requiring a TAV-in-SAV procedure (AUC: 0.943). Additionally, in TAV-in-SAV patients, the risk of coronary obstruction is related to the surgical prosthesis type in place and considerably higher in stented prostheses with externally mounted leaflets or stentless prostheses than stented prostheses with internally mounted leaflets.
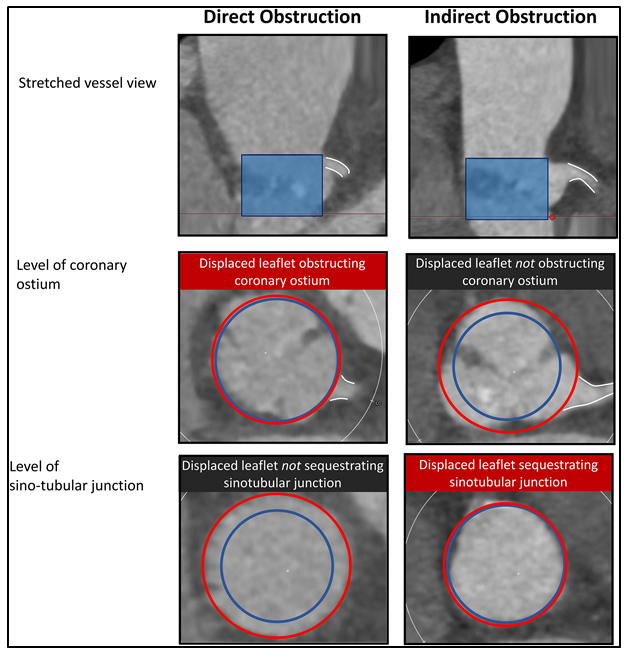
Mechanism of coronary obstruction complicating transcatheter aortic valve implantation. Figures reproduced from Pilgrim and Tomii. JACC Cardiovasc Interv 2023;16(4)426-428.
In patients deemed at high risk for coronary obstruction, coronary protection should be considered (Figure 28). Chimney stenting consists of placing guidewires and an undeployed balloon or stent in the coronary artery. If coronary occlusion is impending or occurs, the stent can be pulled back and deployed in a “chimney” fashion to maintain coronary patency. BASILICA (Bioprosthetic or native Aortic Scallop Intentional Laceration to Prevent Iatrogenic Coronary Artery Obstruction) is an alternative strategy to prevent coronary obstruction. In brief, an electrified guidewire is used to lacerate the aortic valve leaflet that would otherwise obstruct the coronary ostium to preserve coronary inflow. Although the feasibility of the technique has been shown in several studies, the procedures are technically demanding and should be reserved to specialized centres. The ShortCut (Pi-Cardia, Rehovot, Israel) is the first dedicated transcatheter device designed to modify valve leaflets by mechanical splitting. Briefly, the procedure is performed under general anaesthesia using guidance by transoesophageal echocardiography. The device is introduced under fluoroscopic guidance over a standard 0.035-inch guidewire. After crossing the aortic valve, the device is unsheathed to automatically open the positioning arm above the aortic valve. A deflection mechanism allows the device to centre in the valve. Predefined fluoroscopic views are then used to rotate and advance the positioning arm to accurately split the target leaflet at the base. After confirming positioning arm location, the splitting element is activated to puncture the leaflet base from the ventricular aspect. The catheter is then gently retracted, performing a vertical split of the leaflet upward from base to tip. If required, the positioning arm is then rotated towards a second target leaflet and the splitting procedure is repeated (dual split). Afterwards, the ShortCut device is removed, and TAVI is performed using the same guidewire. In the prospective Shortcut study, 60 patients with failed bioprosthetic aortic valves who were at high risk for coronary obstruction after TAVI underwent leaflet modifications using the ShortCut device. The primary efficacy endpoint was the per-patient leaflet splitting success and was achieved in all patients. The primary safety endpoint of procedure-related mortality or stroke at discharge or 7 days was achieved in 98.3% of patients. At 30 days, freedom from coronary obstruction was 95%. The use of cerebral embolic protection devices is frequent in TAVI procedures involving leaflet modification techniques due to the nature and complexity of such interventions.
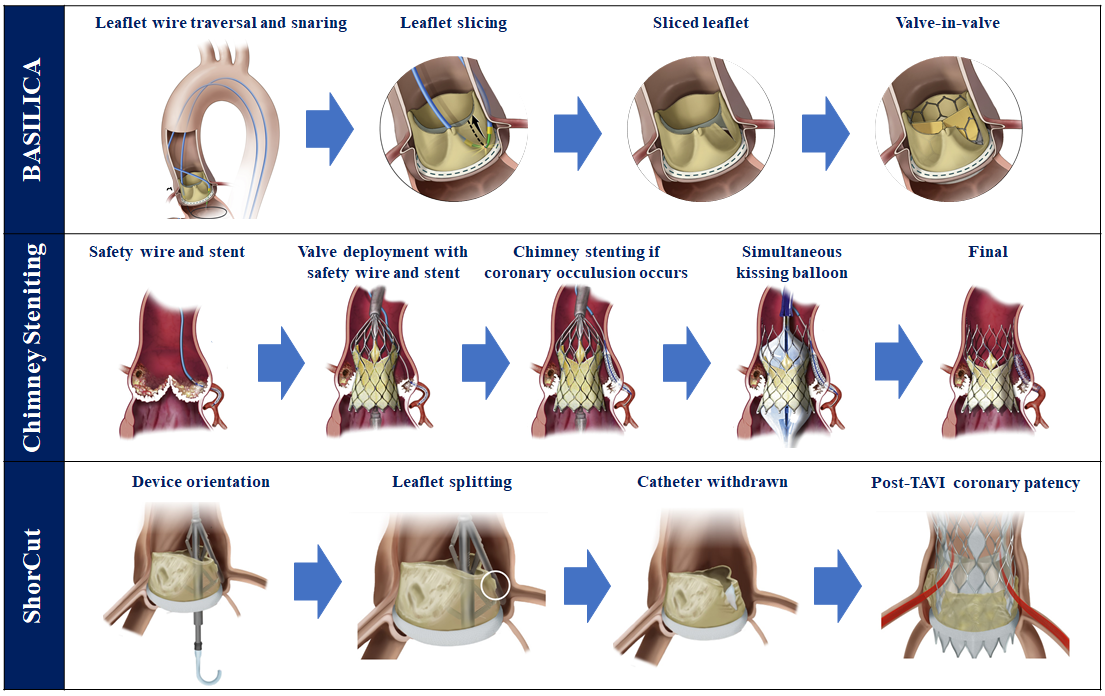
Coronary protection techniques. Figures reproduced and modified from Khan et al. JACC Cardiovasc Interv 2018;11(7):677-689, Mercanti et al. JACC Cardiovasc Interv 2020;13(6):751-761, and Dvir et al. JACC Cardiovasc Interv 2023;16(1):94-102; and Prendergast et al. Eur Heart J 2024;45:3042-3044.
Valve dislocation/embolization occurs in <1% and is accentuated in case of non-calcified native aortic valve leaflets, eccentric and asymmetric calcifications, pre-existing AR, and acute aortic angulation. Valve dislocations are either towards the aorta or into the LV cavity. Although it usually occurs immediately during or after THV implantation, late device migration (up to 1 year after TAVI) has been reported. In an international retrospective registry, valve dislocation/embolization occurred in 273 out of 29,636 patients (0.92%), of which 217 (79.5%) were into the aorta and 56 (20.5%) into the left ventricle. Bailout measures included repositioning attempts using snares or miscellaneous tools, multiple valve implantation (83.2%), and conversion to surgery (19.0%). Of note, successful reposition was achieved in only 51.8% of cases. In a 1:4 propensity score matching analysis, valve dislocation/embolization was associated with an increased risk of stroke at 30 days (10.6% vs. 2.8%, P <0.001) and mortality at 30 days (18.6% vs. 4.9%, P <0.001) and at 1 year (30.5% vs. 16.6%, P <0.001).
New conduction disturbances, including LBBB and high-degree atrioventricular block, requiring new permanent pacemaker implantation, are common adverse events in patients undergoing TAVI. In RCTs, the incidence of new permanent pacemaker implantation and LBBB was higher in patients undergoing TAVI than in those undergoing SAVR, and the development of conduction disturbances is usually more frequent among self-expanding TAVI devices. The development of new conduction disturbances usually occurs within 24 hours, while it may be observed later than 48 hours after TAVI or even after discharge. Pre-existing right bundle branch block, short membranous septum length, non-coronary cusp device landing zone calcification, and deep implantation of the THV have been identified as independent predictors of new conduction disturbances.
New-onset atrial fibrillation is another common rhythm disorder after TAVI. A combination of inflammation, atrial oxidative stress, and increased sympathetic drive may be causal in the development of atrial fibrillation. A recent meta-analysis of 179 studies with 241,712 patients reported that one in ten patients developed new-onset atrial fibrillation after TAVI.
Both rhythm disorders can affect cardiac anatomical and functional remodeling, potentially impacting prognosis. Conduction disturbances may lead to left ventricular dyssynchrony leading to impaired left ventricular function, lead-induced tricuspid regurgitation, and other pacing lead adverse events (fracture and endocarditis) during long-term follow-up. New-onset atrial fibrillation is associated with an increased risk of mortality, stroke, major or life-threatening bleeding, and new permanent pacemaker implantation, , . Therefore, continued monitoring by telemetry and potential risk assessment is a key to detect the rhythm disorders, , , particularly in younger patients with longer life-expectancy.
The Co-STAR (Colchicine for Patients With Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement [NCT04870424]) trial evaluated the efficacy of colchicine to reduce the risk of new-onset arrhythmias after TAVI. In this trial, 120 patients with severe AS undergoing TAVI were randomly allocated in a 1:1 ratio to treatment with colchicine (0.5 mg once daily) or placebo for the duration of 14 days starting the day before TAVI. At 30 days, the primary composite endpoint of new-onset atrial fibrillation or atrioventricular conduction disturbances requiring new permanent pacemaker implantation occurred in 6 patients (10%) in the colchicine group and in 15 patients (25%) in the placebo group (risk difference -15%, 95% CI -23.3 to -1.7, P = 0.031), while there was no significant difference in the incidence of the components between groups. Of note, this trial was prematurely terminated following the recommendation of the Data Safety Monitoring Board after review of the pre-specified interim analysis after complete follow-up of 120 patients, and the the primary outcome event rates were lower than expected. Further studies are warranted to corroborate the effect of anti-inflammatory treatment on the incidence of arrhythmias after TAVI.
Vascular complications typically include dissection, stenosis, perforation, and pseudoaneurysm or aneurysm. Although the incidence has decreased over time (1.5% to 4% in the recent low-risk trials) due to smaller and more flexible delivery systems and increasing operator experience, major vascular complications are still common and associated with increased morbidity and mortality, , , . The most frequent cause is closure device failure leading to continued bleeding, stenosis or vessel closure. In addition to the accurate risk assessment during the preprocedural period, familiarity with closure devices and bailout strategies for iliofemoral vascular complications can mitigate the negative impact of vascular complications. Recently, the TAVI-MultiCLOSE study demonstrated the safety and efficacy of a novel algorithm for percutaneous closure of large-bore arterial access. This algorithm includes the reinsertion of a 4-8 Fr sheath following an initial closure with one or two suture-based vascular closure devices, which provides the opportunity to perform an angiographic control and tailor the final vascular closure with either an additional suture- or plug-based vascular closure device, or neither of these. In this study, the reported incidence of minor and major vascular complication with the consecutive use of this algorithm was 2.2% and 0.6%, respectively.
Stroke remains one of the most feared complications of TAVI and is associated with significant morbidity and mortality. TAVI-related stroke can be differentiated by aetiology and timing, . Acute stroke, the most common type of TAVI-related stroke, occurs during or within 24 hours of the procedure and is related to dislodged atheroma and aortic valve debris. After the acute phase, new-onset atrial fibrillation and bioprosthetic thrombosis may increase the risk of subacute (24 hours to 30 days) and early (>30 days to 1 year) stroke. In the late period (>1 year after TAVI), the risk of stroke is dominated by atherosclerotic burden rather than procedural features, and the incidence of late stroke in the TAVI population is comparable to that in the general population. Although there is no robust evidence that cerebral embolic protection devices prevent cerebrovascular events during the procedure, their use may be considered in patients with high-risk TAVI, such as calcified aortic valve and valve-in-valve TAVI with leaflet modification techniques (BASILICA and ShortCut). In addition, optimal anticoagulation during and after the procedure is essential to prevent stroke. Once neurological events are suspected, patients should be promptly evaluated by a neurologist and undergo diagnostic imaging whenever indicated. Neurointervention, including mechanical thrombectomy and thrombolytic therapy may be required.
Patients undergoing TAVI are predominantly elderly and feature both thromboembolic and bleeding risks. Optimal antithrombotic therapy after TAVI has been debated for the past decade (Table 14). In patients with no indication for anticoagulation therapy, lifelong single antiplatelet therapy (SAPT) is the standard treatment recommendation, as dual antiplatelet therapy (DAPT) is associated with an increased risk of bleeding complications without benefit in terms of thromboembolic event protection, , , .
Table 14. Randomized clinical trials on antithrombotic therapy after TAVI.
|
Trial First author |
Strategy |
Population |
Primary end point |
Period |
Main Result |
|---|---|---|---|---|---|
|
No indication for anticoagulant therapy |
|||||
|
Antiplatelet therapy |
|||||
|
Ussia et al. |
3 mo DAPT (clopidogrel + aspirin) |
79 patients without underlying indication for OAC or recent stent implantation |
Composite of death, MI, major stroke, life-threatening or major bleeding, or urgent conversion to surgery |
6 mo |
No significant difference for SAPT vs. DAPT (15% vs. 18% respectively; P = 0.85) |
|
SAT-TAVI |
6 mo DAPT (clopidogrel or ticlopidine + aspirin) |
120 patients without underlying indication for OAC or recent stent implantation |
VARC-2 safety end points |
30 d |
No difference on the primary endpoint. Composite of major and minor vascular complication more frequent with DAPT (13.3% vs. 5%; P<0.05) |
|
ARTE |
3 mo DAPT (clopidogrel + aspirin or acetylsalicylic acid) |
222 patients without underlying indication for OAC or recent stent implantation |
Composite of death, MI, stroke or TIA, or life-threatening or major bleeding |
3 mo |
15.3% vs. 7.2% for DAPT and SAPT respectively (OR 2.31, 95% CI 0.95 to 5.62, P = 0.065) |
|
POPular TAVI cohort A |
SAPT (aspirin) |
665 patients without an indication for long-term anticoagulation |
The two primary end point were all bleeding and non–procedure-related bleeding |
12 mo |
Both all bleeding and non-procedure bleeding more frequent with DAPT (26.6% vs. 15.1%; P = 0.001, 24.9% vs. 15.1%; P = 0.005, respectively).
|
|
Oral anticoagulation |
|||||
|
GALILEO |
Rivaroxaban group (rivaroxaban + 3 mo aspirin |
1,644 patients without indication for long-term OAC |
Composite of death or thromboembolic events. |
24 mo |
Rivaroxaban group had higher rates of death or thromboembolic events (HR 1.35, 95% CI 1.01 to 1.81; P = 0.04) |
|
Major, disabling, or life-threatening bleeding |
Rivaroxaban group hadhigher rates of bleeding event (HR 1.50, 95% CI 0.95 to 2.37, P = 0.08) |
||||
|
Low Risk Trial 2.0 |
Warfarin + Aspirin for 30 days |
94 patients without indication for OAC |
Composite of HALT, at least moderate RLM, haemodynamic dysfunction (mean aortic valve gradient ≥20 mmHg, effective orifice area ≤1.0 cm2, dimensionless valve index <0.35, or moderate or severe aortic regurgitation), stroke, or TIA |
30 days |
7.0% vs. 26.5% for Warfarin + Aspirin and Aspirin alone, respectively (OR 4.8, 95% CI 1.3 to 18.3, P = 0.014) |
|
ATLANTIS stratum 2 |
Apixaban |
1049 without indication for long-term OAC |
Composite of death, MI, stroke or TIA, systemic embolism, intracardiac or bioprosthesis thrombosis, DVT or PE and life-threatening, disabling, or major bleeding |
12 mo |
16.9% vs. 19.3% (HR 0.88, 95% CI 0.66 to 1.17) |
|
Major, disabling, or life-threatening bleeding |
7.8% vs. 7.3% (HR 1.09, 95% CI 0.70 to 1.69) |
||||
|
ADAPT-TAVR |
Edoxaban |
229 patients without indication for long-term OAC |
Leaflet thrombosis on 4-dimensional computed tomography |
6 mo |
9.8% vs. 18.4% (absolute difference −8.5%; 95% CI −17.8% to 0.8%; P = 0.076) |
|
Indication for anticoagulant therapy |
|||||
|
POPular TAVI cohort B |
OAC |
326 patients with indication for long-term OAC |
All bleeding and non–procedure-related bleeding |
12 mo |
Both all bleeding and non-procedure bleeding more frequent with OAC with clopidogrel (34.6% vs. 21.7%; P = 0.01, 34.0% vs. 21.7%, P = 0.02, respectively) OAC alone was non-inferior in terms of the thromboembolic composite endpoint of cardiovascular death, ischemic stroke, or myocardial infarction (13.4% vs. 17.3%, difference -3.9 percentage points, 95% CI for non-inferiority -11.9 to 4.0) |
|
ENVISAGE-TAVI AF |
Edoxaban |
1426 patients with indication for long-term OAC |
Death, MI, stroke, systemic thromboembolic event valve thrombosis, or major bleeding |
Median 554 and 530 days |
17.3 per 100 person-years vs. 16.5 per 100 person-years (HR 1.05, 95% CI 0.85 to 1.31) |
|
Major bleeding |
9.7 per 100 person-years vs. 7.0 per 100 person-years (HR 1.40, 95% CI 1.03 to 1.91) |
||||
|
ATLANTIS stratum 1 |
Apixaban |
451 patients with indication for long-term OAC |
Composite of death, MI, stroke or TIA, systemic embolism, intracardiac or bioprosthesis thrombosis, DVT or PE, and life-threatening, disabling, or major bleeding |
12 mo |
22% vs. 21.9% (HR 1.02, 95% CI 0.69 to 1.51) |
|
Major, disabling, or life-threatening bleeding |
10.3% vs. 11.4% (HR 0.91, 95% CI 0.52 to 1.60) |
||||
|
CI = confidence interval; DAPT = dual antiplatelet therapy; DVT = deep vein thrombosis; HALT = hypoattenuated leaflet thickening; HR = hazard ratio; MI = myocardial infarction; OAC = oral anticoagulation; OR = odds ratio; PE = pulmonary embolism; RLM = reduced leaflet motion; SAPT = single antiplatelet therapy; TIA = transient ischemic attack ; VARC =valve academic research criteria. |
|||||
Therefore, temporal DAPT is considered only in patients at low risk of bleeding or in those with recent coronary stenting (Table 15), .
Table 15. Guideline recommendations: Antithrombotic therapy after TAVI.
|
2020 AHA/ACC Valvular Heart Disease Guideline |
2021 ESC/EACTS Valvular Heart Disease Guideline |
|||||
|---|---|---|---|---|---|---|
|
Recommendations |
COR |
LOE |
Recommendations |
COR |
LOE |
|
|
No indication for oral anticoagulant |
||||||
|
Single antiplatelet therapy with aspirin (75-100mg daily). |
2a |
B-R |
Lifelong single antiplatelet therapy. |
I |
A |
|
|
For patients at low risk of bleeding, DAPT (aspirin and clopidogrel) may be reasonable for 3-6 months after TAVI. |
2b |
B-NR |
If recent coronary stenting (<3 months), consider DAPT (aspirin and clopidogrel) for 1-6 months, and then single antiplatelet therapy (aspirin or clopidogrel) lifelong. |
NA |
||
|
For patients at low risk of bleeding, OAC with VKA may be reasonable for at least 3 months after TAVI. |
2b |
B-NR |
||||
|
Routine use OAC is not recommended after TAVI. |
III |
B |
||||
|
Concomitant indication for oral anticoagulant |
||||||
|
No recommendation made in ACC/AHA guidelines. |
Lifelong OAC therapy. |
I |
B |
|||
|
If recent coronary stenting (<3 months) continue OAC lifelong and consider a single antiplatelet drug (aspirin or clopidogrel) for 1-6 months. |
NA |
|||||
|
ACC/AHA = American College of Cardiology/American Heart Association; AS = aortic stenosis; COR = class of recommendation; DAPT = dual antiplatelet therapy; ESC/EACTS = European Society of Cardiology and the European Association for Cardio-Thoracic Surgery; OAC = Oral anticoagulant; TAVI = transcatheter aortic valve implantation; VKA = vitamin K antagonist. |
||||||
If there is an established indication for DAPT, the antithrombotic management should follow the recommendations for this indication. The NAPT (Non-antithrombotic Therapy After Transcatheter Aortic Valve Implantation [NCT06007222]) trial is currently underway to evaluate the feasibility of no antiplatelet therapy after successful TAVI in patients without an indication for OAC and an indication for antiplatelet therapy other than TAVI. Of note, the study duration will limit mid-term follow-up (up to 3 years) and assessment of valve function, including the presence of subclinical valve thrombosis, is only planned at 6 months and 1 year.
Patients with THV have a lifelong risk for bioprosthetic thrombosis. Subclinical leaflet thrombosis after TAVI, which can manifest as HALT and/or reduced leaflet motion (RLM) has been the focus of intense clinical research. Although there are conflicting data regarding the incidence, natural history, and clinical impact of subclinical leaflet thrombosis, the prevention of subclinical leaflet thrombosis has been emphasized as it may impact bioprosthetic durability and the risk of stroke, , , , , , , , . Several studies have reported that OAC is associated with a reduced risk of subclinical leaflet thrombosis, , . In the CT substudy of the GALILEO trial, rivaroxaban added to aspirin was associated with a lower incidence of RLM grade ≥3 and leaflet thickening at 90-day CT follow-up compared with clopidogrel plus aspirin (2.1% vs. 10.9%, P = 0.01, and 12.4% vs. 32.4%). Similarly, a CT-substudy of the ATLANTIS trial reported that apixaban reduced the risk of subclinical obstructive valve thrombosis (defined as ≥1 prosthetic valve leaflet with grade 3 or 4 RLM or grade 3 or 4 HALT) compared with antiplatelet therapy (odds ratio: 0.51, 95% CI 0.30-0.86) but not compared with vitamin K antagonists (VKAs) (odds ratio: 1.80; 95% CI: 0.62-5.25). However, RCTs comparing OAC with antiplatelet therapy have consistently shown that OAC is associated with an increased risk of bleeding and death compared with antiplatelet therapy, . Therefore, current ESC/EACTS guidelines do not recommend routine use of OAC in patients with no indication for OAC (Class III) (Table 15).
In patients with an established indication for OAC, OAC should be continued after TAVI and additional antiplatelet therapy is generally not recommended. In the POPular TAVI trial cohort B, the addition of clopidogrel to OAC was associated with an increased risk of both all bleeding and non-procedure bleeding (34.6% vs. 21.7%; P = 0.01, 34.0% vs. 21.7%; P = 0.02, respectively), and OAC alone was non-inferior with respect to the thromboembolic composite endpoint of cardiovascular death, ischemic stroke, or myocardial infarction (13.4% vs. 17.3%, difference -3.9 percentage points, 95% CI for non-inferiority -11.9 to 4.0). Whether these patients should be treated with DOACs or VKA remains a subject of debate. In the ENVISAGE-TAVI AF trial, edoxaban was non-inferior to VKAs in terms of the primary efficacy composite outcome of death from any cause, myocardial infarction, ischemic stroke, systemic thromboembolism, valve thrombosis, or major bleeding (HR 1.05; 95% CI 0.85-1.31, Pnon-inferiority = 0.01). However, the primary safety endpoint of major bleeding occurred more frequently in the edoxaban group compared with VKA (HR 1.40, 95% CI 1.03-1.91). Similarly, the ATLANTIS trial Stratum 1 reported no difference in the primary composite endpoint of death, myocardial infarction, stroke or transient ischaemic attack, systemic embolism, intracardiac or bioprosthesis thrombosis, deep vein thrombosis or pulmonary embolism, and life-threatening, disabling, or major bleeding at 1 year and in the primary safety endpoint of major, disabling, or life-threatening bleeding (HR 0.91; 95% CI 0.52-1.60) between patients treated with apixaban and those receiving VKA.
Data on the durability of THV have been reported from the randomized clinical trials and large scale real-world registries. However, the durability data for THV are currently limited to 10-year follow-up and are mainly derived from elderly populations, , , , . Among surgical studies monitoring durability, structural valve deterioration (SVD) has mainly occurred more than 10 years after implantation although these studies used clinical rather than echocardiographic follow-up (Figure 29). The available evidence on the durability of THV is somewhat confounded due to varying definitions and surrogates of BVD, and a substantial competitive risk of death especially in the cohorts of early TAVI trials and registries, which has variably been accounted for in previous studies. The definition of SVD and BVD has changed over time to incorporate the underlying pathology, haemodynamic significance, and clinical sequelae. Several pathologies can lead to BVD, which have been clustered into SVD and non-SVD etiologies, in addition to two additional entities including thrombosis and endocarditis according to the European Association of Percutaneous Cardiovascular Interventions (EAPCI) and the VARC definitions, . The key difference between the definitions is the need for haemodynamic criteria for the assessment of BVD severity. VARC-3 stratifies BVD according to the haemodynamic significance into morphological (without significant haemodynamic valve deterioration), and haemodynamically significant valve deterioration: moderate (Stage 2) and severe (Stage 3) HVD. Contrary to previous definitions, VARC-3 incorporates temporal dynamic changes of the echocardiographic haemodynamic parameters of the aortic valve prosthesis rather than using fixed reference values as diagnostic cut-off thresholds for HVD. Furthermore, the VARC-3 definition considers three stages of BVF: Stage 1 includes any BVD with clinically expressive criteria such as new-onset or worsening symptoms, left ventricular dilatation/hypertrophy/dysfunction, or pulmonary hypertension, or irreversible Stage 3 HVD; Stage 2 includes aortic valve reintervention; and Stage 3 includes valve-related death (Table 16).
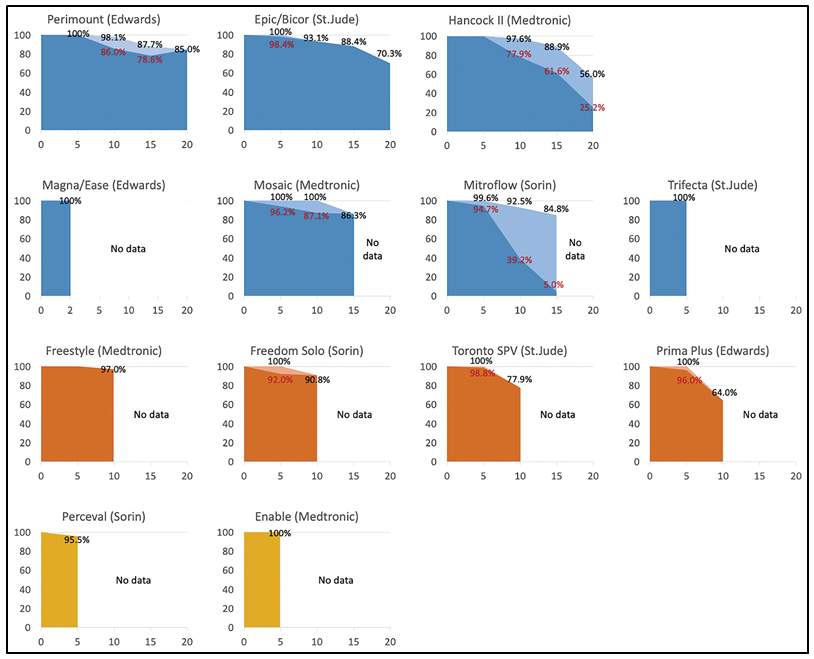
Valve durability according to the type of surgical bioprosthesis.
Figures reproduced from Windecker et al. Eur Heart J 2022;43(29):2729-2750.
Table 16. Definitions of structural valve deterioration and bioprosthetic valve failure.
|
ESC/EAPCI/EACTS |
VARC-3 |
||
|---|---|---|---|
|
Structural valve deterioration (SVD) |
|||
|
Any of following: Leaflet integrity abnormality (i.e. torn or flail causing intra-frame regurgitation); Leaflet structure abnormality(i.e. pathological thickening and/or calcification causing valvular stenosis or central regurgitation); Leaflet function abnormality (i.e. impaired mobility resulting in stenosis and/or central regurgitation); Strut/frame abnormality (i.e. fracture) |
Morphological SVD |
Morphological valve deterioration |
Intrinsic permanent changes to the prosthetic valve, including leaflet tear, disruption, flail leaflet, leaflet fibrosis and/or calcification without significant haemodynamic changes. |
|
Any of following: Mean transprosthetic gradient ≥20mmHg and <40 mmHg; Mean transprosthetic gradient ≥10 and <20 mmHg change from baseline; Moderate intra-prosthetic aortic regurgitation, new or worsening (>1+/4+) from baseline |
Moderate haemodynamic SVD |
Moderate haemodynamic valve deterioration |
Morphological valve deterioration AND: Increase in mean transvalvular gradient ≥10 mmHg resulting in mean gradient ≥20 mmHg‡ with concomitant decrease in AVA ≥0.3 cm2 or ≥25% and/or decrease in Doppler velocity index ≥0.1 or ≥20% compared to echocardiographic assessment performed 1 to 3 months post-procedure (or discharge if not available), |
|
Any of following: Mean transprosthetic gradient ≥40 mmHg; Mean transprosthetic gradient ≥20 mmHg change from baseline; Severe intra-prosthetic aortic regurgitation, new or worsening (>2+/4+) from baseline |
Severe haemodynamic SVD |
Severe haemodynamic valve deterioration |
Morphological valve deterioration AND: Increase in mean transvalvular gradient ≥20 mmHg resulting in mean gradient ≥30 mmHg‡ with concomitant decrease in AVA ≥0.6 cm2 or ≥50% and/or decrease in Doppler velocity index ≥0.2 or ≥40% compared to echocardiographic assessment performed 1 to 3 months post-procedure (or discharge if not available), |
|
haemodynamic and morphological SVD |
|||
|
Bioprosthetic Valve failure (BVF) |
|||
|
- Autopsy findings of bioprosthetic valve dysfunction, likely related to the cause of death, or valve-related death; - Repeat intervention following confirmed diagnosis of bioprosthetic valve dysfunction; - Severe haemodynamic SVD |
Stage 1 |
Any significant bioprosthetic valve dysfunction with clinically expressive criteria (new-onset or worsening symptoms, left ventricular dilation/ hypertrophy/ dysfunction, or pulmonary hypertension) |
|
|
Stage 2 |
Aortic valve reoperation or reintervention |
||
|
Stage 3 |
Valve-related death |
||
|
‡ This criteria for haemodynamic dysfunction assumes normal flow. † Cardiovascular mortality presumed to be associated with bioprosthetic valve dysfunction. AR = aortic valve regurgitation; AVA = aortic valve area; EAPCI/ ESC/EACTS =European Association of Percutaneous Cardiovascular Interventions/European Society of Cardiology/European Association for Cardio-Thoracic Surgery; LV = left ventricular; VARC = Valve Academic Research Consortium. |
|||
Available data on the durability comparing SAVR with TAVI using the VARC-3 definition are limited, but recent reports from RCTs are increasingly adapting the VARC-3 definition (Figure 30). In the pooled analysis of the PARTNER 2A trial and the SAPIEN 3 registry, the SAPIEN-XT TAVI cohort had a significantly higher 5-year exposure adjusted incidence rates (per 100 patient-years) of SVD (1.61 ± 0.24% vs. 0.63 ± 0.16%), SVD-related BVF (0.58 ± 0.14% vs. 0.12 ± 0.07%), and all-cause (structural or nonstructural) BVF (0.81 ± 0.16% vs. 0.27 ± 0.10%) (P <0.01 for all) compared with SAVR, while there was a similar rate of SVD (0.68 ± 0.18% vs. 0.60 ± 0.17%; P = 0.71), SVD-related BVF (0.29 ± 0.12% vs. 0.14 ± 0.08%; P = 0.25), and all-cause BVF (0.60 ± 0.15% vs. 0.32 ± 0.11%; P = 0.32) between SAPIEN 3 TAVI and SAVR. The PARTNER 3 trial also reported on the durability data of the SAPIEN 3 THV. At 5 years, the Kaplan–Meier estimates of BVF of any cause were 3.3% in the TAVI group and 3.8% in the surgery group and the incidence of SVD-related BVF was 1.4% in the TAVR group and 2.0% in the surgery group. Favourable durability data have also been reported for self-expanding CoreValve/Evolut THV In the pooled data of the CoreValve US High Risk Pivotal (n = 726) and SURTAVI (n = 1,618) RCTs, the cumulative incidence of VARC-3 BVD and SVD (moderate or severe HVD) treating death as a competing risk was lower in patients undergoing TAVI than surgery (BVD: 9.7% vs. 15.3%, sHR 0.57; 95% CI 0.45-0.73, P <0.001; SVD: 2.4% vs. 4.5%, sHR 0.54, 95% CI 0.33-0.88, P = 0.01, respectively). The NOTION trial reported the longest durability data to date on the self-expanding CoreValve THV compared with SAVR. In this trial, severe SVD (VARC-3 severe HVD) was recorded in 1.5% and 10.0% (HR 0.2; 95% CI 0.04–0.7; P = 0.02) of surviving patients after TAVI and SAVR, respectively. The cumulative incidence for severe non-SVD BVD was 20.5% and 43.0% (P <0.001) and for endocarditis 7.2% and 7.4% (P = 1.0) after TAVI and SAVR, respectively, and no patient experienced clinical valve thrombosis. BVF occurred in 9.7% of TAVI and 13.8% of SAVR patients (HR 0.7; 95% CI 0.4–1.5; P = 0.4). Although RCTs have provided high quality data on the durability of THV, comparisons with the durability of SAVR should consider the non-uniform use of surgical bioprostheses and increased post-procedural gradients immediately after implantation. In summary, available data suggest similar durability of THV and surgical bioprostheses up to 10 years after AVR.
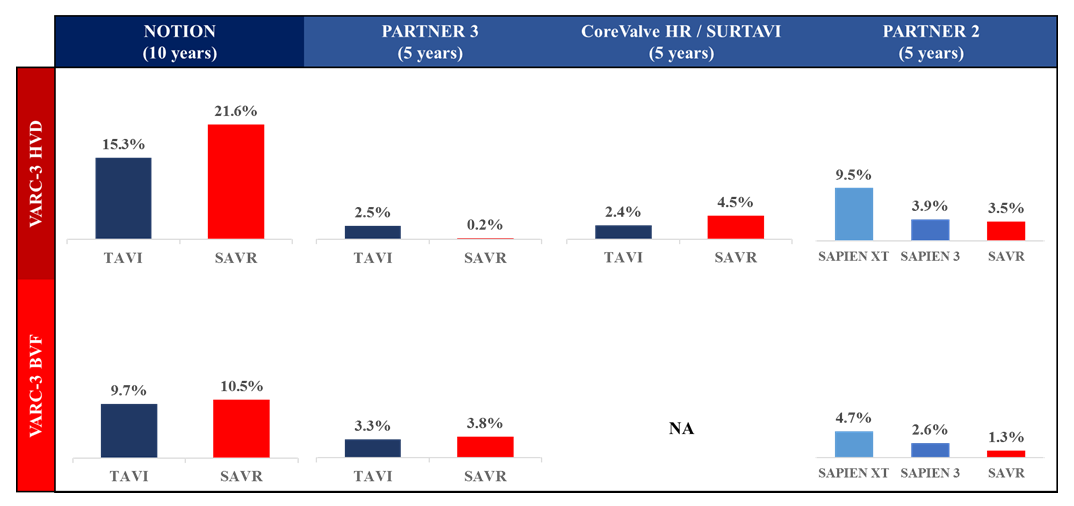
Valve durability comparing transcatheter and surgical bioprostheses in ranodomized control trials.
Data reproduced from Thyregod et al. Eur Heart J 2024;45:1116-1124; Mack et al. N Nngl J Med 2023;389:1949-1960; Yakubo et al. J Am Coll Cardiol 2025. [Online ahead of print]. doi: 10.1016/j.jacc.2025.02.009; and Pibarot et al. J Am Coll Cardiol 2020;76:1830-1843.
Bioprosthetic valve thrombosis is relatively rare but an important cause of BVD. Traditionally, bioprostheses have been considered less thrombogenic than mechanical heart valves. However, recent evaluations of the performance and durability of bioprostheses and the advent of MDCT as a novel diagnostic modality has highlighted the issue in various types of THV as well as SAVR valves. Bioprosthetic valve thrombosis typically comprises two distinct entities, clinical valve thrombosis and subclinical leaflet thrombosis. Clinical valve thrombosis is defined by the detection of valve dysfunction secondary to thrombosis or a mobile mass detected on the prosthesis typically associated with increased transvalvular gradients. For the treatment of clinical valve thrombosis, current ESC/EACTS and AHA/ACC guidelines for the management of valvular heart disease recommend OAC with VKA or unfractionated heparin as the first-line treatment before considering invasive treatment (Table 17). After a period of 3 to 6 months, OAC may be discontinued if resolution of thrombus and improvement in bioprosthetic function are achieved. In case of unresolved or recurrent clinical valve thrombosis, lifelong OAC should be considered, .
Table 17. Guideline recommendations: Treatment of clinical valve thrombosis.
|
2020 AHA/ACC Valvular Heart Disease Guideline |
2021 ESC/EACTS Valvular Heart Disease Guideline |
|||||
|---|---|---|---|---|---|---|
|
Recommendations |
COR |
LOE |
Recommendations |
COR |
LOE |
|
|
In patients with suspected or confirmed bioprosthetic valve thrombosis who are haemodynamically stable and have no contraindications to anticoagulation, initial treatment with a VKA is reasonable. |
2a |
B-NR |
Anticoagulation using a VKA and/or UFH is recommended in bioprosthetic valve thrombosis before considering re-intervention. |
I |
C |
|
|
Anticoagulation should be considered in patients with leaflet thickening and reduced leaflet motion leading to elevated gradients, at least until resolution. |
IIa |
B |
||||
|
ACC/AHA = American College of Cardiology/American Heart Association; COR = class of recommendation; ESC/EACTS = European Society of Cardiology and the European Association for Cardio-Thoracic Surgery; UFH = unfractionated heparin; VKA = vitamin K antagonist. |
||||||
In contrast to clinical valve thrombosis, subclinical leaflet thrombosis (HALT and RLM) is most often an incidental finding as assessed by MDCT. The epidemiology and natural history of clinical and subclinical valve thrombosis is not well established. MDCT assessment of bioprostheses in the framework of the RCTs have reported that subclinical leaflet thrombosis occurs in 10-50% of patients after TAVI and may be associated with an increased risk of clinical valve thrombosis. However, subclinical valve thrombosis may also regress spontaneously without changes in antithrombotic therapy, complicating the establishment of a uniform treatment strategy, . In this context, close clinical and echocardiographic follow-up is recommended with use of OAC being individualized depending on HALT/RLM evolution and bleeding risk.
Prosthetic valve endocarditis is a rare but serious complication associated with valve failure and mortality after TAVI. The incidence of prosthetic valve endocarditis after TAVI is comparable to that after SAVR. In PARTNER 1 and 2, the incidence of prosthetic valve endocarditis was 5.21 per 1,000 person-years (95% CI, 4.26-6.38) for TAVI and 4.10 per 1,000 person-years (95% CI, 2.33-7.22) for SAVR during a mean follow-up of 2.69 ± 1.55 years (95% CI, 4.19–6.12). Similarly, in the pooled analysis from 3 RCTs (CoreValve US High Risk, SURTAVI, and Evolut Low Risk), the cumulative incidence of prosthetic valve endocarditis was 1.01% (95% CI, 0.47%–1.96%) after TAVI and 1.58% (95% CI, 0.97%–2.46%) after SAVR (P = 0.047) at 5 years. The risk of prosthetic valve endocarditis is most pronounced during the periprocedural period. The nationwide SwissTAVI registry reported that the incidence for periprocedural, early, and late endocarditis after TAVI was 2.59, 0.71, and 0.40 events per 100 person-years, respectively. Across studies, prosthetic valve endocarditis confers a higher mortality, , , , and 20% of TAVI endocarditis cases require cardiac surgery, which is generally complex and associated with morbidity. Therefore, optimal antibiotic coverage and procedural infection prevention using surgical standards as well as antibiotic prophylaxis for invasive procedures after TAVI are key to mitigate the risk of prosthetic valve endocarditis with TAVI.
With the expansion of TAVI indications, there is an ever increasing demand for TAVI. In a population-level retrospective cohort study of 22,876 referrals for AVR in Toronto, Ontario, there was a significant increase in wait times for AVR between 2012 and 2018 (from a median of 40 days in 2012 to 90 days in 2018), which was more pronounced for TAVI (increase from a median 80 days in 2012 to 110 days in 2018), and associated with increased mortality and heart failure-related hospitalizations while on the waiting list. The UK Valve for Life initiative is a national project to improve the delivery of transcatheter valve therapy. In the national survey of all TAVI centres in England, 52% of centres reported that they could not perform more TAVI due to cath lab/hybrid room capacity limitations, lack of coronary care unit or ward bed capacity, and MDCT scan availability, .
Against the background of limited space and human resources, several approaches have been developed to improve access to TAVI. The minimalist approach, including local anaesthesia without transesophageal echocardiographic guidance to simplify transfemoral TAVI procedures is now widely used worldwide. In addition to the minimalist approach, TAVI using fast-track pathways is receiving increasing attention to reduce the length of hospital stay, leading to early discharge after TAVI. The Vancouver 3M (Multidisciplinary, Multimodality, but Minimalist) Clinical Pathway and the FAST-TAVI Pathway, which focused on early and next-day discharge through the use of objective anatomic and functional screening criteria and streamlined, standardized peri-procedural and postprocedural management guidelines, have shown that implementation of the fast-track pathways allow a high rate of next-day or early home discharge after TAVI without increasing the risk of adverse events in carefully selected patients, , . More recently, the Cleveland Clinic has reported the safety and feasibility of same-day discharge following transfemoral TAVI compared with next-day discharge in the contemporary TAVI era. The same-day discharge was based on 6 criteria including procedural characteristics and outcomes, patient comfort, and availability of social support after discharge. In 2020, same-day discharge and next-day discharge accounted for 22.1% (n = 114 of 516) and 63.8% (n = 329 of 516) of transfemoral TAVI, respectively, and there was no significant difference in in-hospital clinical outcomes and 30-day readmissions. FAST-TAVI II was a prospective, multicentre, cluster-randomized, controlled trial in patients with severe symptomatic AS to evaluate the efficacy and safety of a dedicated training program aimed at reducing the length of hospital stay after transfemoral TAVI. In this cluster-randomized trial involving 20 French TAVI centres, patients randomized to the intervention group received a dedicated training programme implementing 10 quality of care measures to reduce length of hospital stays, with an implementation phase of eight weeks. The primary endpoint was the proportion of patients discharged early within 3 days which was achieved more frequently in the intervention group than the control group (58.1% vs. 42.3%, P <0.0001). In summary, minimalist TAVI with fast-track pathways has demonstrated a reduction in the length of hospital stay after TAVI without increasing the risk of adverse events. Further efforts are warranted to reduce the length of hospital stay and increase the proportion of patients discharged home early after TAVI.
The choice of prosthesis type in patients referred for valve replacement remains a debated issue, particularly in younger patients with a longer life expectancy. Although several studies have consistently shown that mechanical prostheses are associated with a lower risk of reoperation than bioprostheses, the benefit of extended durability intrinsic to mechanical prostheses must be balanced against the lifelong need for OAC and the associated risk of bleeding and the inconvenience of monitoring, dietary restrictions, medication interactions, and the need to restrict participation in some types of athletic activity. Current guidelines recommend mechanical prostheses in younger patients (patients younger than 50 years of age in AHA/ACC guideline and younger than 60 years of age in ESC/EACTS guideline), . In a recent report from the STS Adult Cardiac Surgery Database comparing isolated mechanical or bioprosthetic SAVR in patients aged 40-75 years (2008-2019), patients 60 years of age or younger treated with mechanical SAVR showed benefit in terms of freedom from risk-adjusted all-cause mortality (40-49 years: stabilized inverse probability weights (sIPW)-adjusted HR 0.69, 95% CI 0.59-0.79; 50-59 years: sIPW-adjusted HR 0.87, 95% CI 0.80-0.94; 60-69 years: sIPW-adjusted HR 0.99, 95% CI 0.91-1.08; and 70-79 years: sIPW-adjusted HR 1.12, 95% CI 0.97-1.29, respectively). However, several studies have reported that the use of mechanical prostheses in surgical procedures has declined over time even in younger patients, and the extended durability of the newer bioprostheses as well as the emergence of TAVI have further expedited this trend, , . Consequently, the number of failed bioprostheses requiring repeat aortic valve intervention has increased. Traditionally, redo SAVR has been the standard procedure for the treatment of failed bioprostheses; however, multiple open-heart surgeries carry an increased risk related to technical issues of reoperation as well as patient’s age and comorbidities. Valve-in-valve TAVI (TAV-in-SAV or TAV-in-TAV) has emerged as a less invasive and preferred treatment option for patients with failed bioprostheses and has markedly increased in use, , .
TAVI for failed surgical bioprosthesis (TAV-in-SAV) is a predominant form of valve-in-valve TAVI. Several dedicated registries have reported procedural and clinical outcomes of TAV-in-SAV, , , , , , . In a meta-analysis of 5,553 TAV-in-SAV cases from 24 studies, the procedural success rate was 97% (95% CI 94-98%) with a low 30-day adverse event rate (death: 5% [95% CI 3-6%]; stroke: 2% [95% CI 1-2%]; myocardial infarction: 1% [95% CI 1-2%]; and new permanent pacemaker implantation: 7% [95% CI 5-11%], respectively). The PARTNER 2 ViV and continued access registries have reported long-term clinical outcomes up to 5 years of follow-up in patients undergoing TAV-in-SAV at high surgical risk (N = 369, mean age was 78.9 ± 10.2 years, and the mean STS-PROM was 9.1 ± 4.7%). At 5 years, all-cause death and stroke occurred in 50.6% and 10.5% of patients, respectively, and a significant improvement in patient health status was observed compared to baseline. The bioprosthetic haemodynamic properties were sustained over 5 years, and the incidence of VARC-3 SVD-related BVF was low (2.3%). The outcome of TAV-in-SAV has been compared to redo SAVR. In a French nationwide propensity-matched analysis of 717 matched pairs (2010-2019), TAV-in-SAV was associated with a lower risk of the composite of all-cause mortality, all-cause stroke, myocardial infarction, and major or life-threatening bleeding at 30 days compared with redo SAVR (odds ratio: 0.62; 95% CI: 0.44-0.88), while the incidence of the combined endpoint was similar between groups during a median follow-up of 516 days, (odds ratio: 1.18; 95% CI: 0.99-1.41). Similarly, in a meta-analysis including a total of 16,207 patients (8,048 TAV-in-SAV and 8,159 redo SAVR) from 35 studies (2015-2020), TAV-in-SAV was associated with lower all-cause mortality and major bleeding at 30 days (odds ratio 0.52, 95% CI: 0.39-0.68; and odds ratio 0.48 95% CI: 0.28-0.80, respectively), but comparable 1-year mortality compared with redo SAVR (odds ratio 0.90, 95% CI: 0.61-1.32). Of note, TAV-in-SAV was associated with a higher rate of severe prosthesis-patient mismatch (OR: 4.63; 95% CI: 3.05-7.03). TAV-in-SAV for small surgical bioprostheses, stenotic cause of bioprosthesis failure, and pre-existing prosthesis-patient mismatch, all of which are associated with an increased risk of severe prosthesis-patient mismatch after TAV-in-SAV, are the principal risk factors for redo aortic valve surgery and impaired survival after TAV-in-SAV, . The use of self-expanding THV and bioprosthetic valve fracture have been suggested to mitigate the risk of prosthesis-patient mismatch after TAV-in-SAV, , .
As TAVI has expanded to low-risk patients with longer life-expectancy, the number of patients with failed THV will increase. Owing to the favourable outcomes of TAV-in-SAV, TAV-in-TAV (redo TAVI) has been considered as a reasonable treatment option for THV failure. In the multicentre Redo-TAVR registry of 63,876 TAVI patients treated at 37 centers in 2019, 212 patients (0.33%) underwent redo TAVI and 138 (64%) were treated 1 year or later after the initial TAVI procedure. Device success according to the VARC-2 definition was achieved in 180 patients (85.1%) with most failures being attributable to high residual gradients (14.1%) or regurgitation (8.9%). Kaplan-Meier survival estimates were 97.2% at 30 days and 86.5% at 1 year, respectively. The STS/ACC TVT registry compared procedural and clinical outcomes and bioprosthetic haemodynamics in 1,320 propensity-matched pairs of patients undergoing redo TAVI and native TAVI (redo TAVI cohort: mean age 78 years; 42.3% female; mean STS-PROM 8.1%). Although mean transprosthetic gradient was higher in the redo TAVI group compared with the native TAVI group (15 mmHg vs. 12 mmHg; P <0.0001), there were no between group differences with respect to risk of moderate or severe PVR, procedural complications, and clinical outcomes at 30 days and 1 year (death, stroke, and valve-related admission).
In addition to the risk of increased residual gradients and patient-prosthesis mismatch after the procedure, valve-in-valve TAVI procedures have an increased risk of coronary obstruction and potentially challenging coronary access after the procedure. The incidence of coronary obstruction following valve-in-valve TAVI is considerably higher than that in TAVI for native aortic valve (2.0-3.5% vs. <1.0%), , due to a Neoskirt potentially extending above the coronary ostia resulting in sinus sequestration. In addition, the risk is pronounced in specific procedures. For example, in TAV-in-SAV procedures, TAVI in failed stentless surgical bioprotheses is more challenging compared with TAVI in failed stented surgical bioprotheses, due to the lack of fluoroscopic markers at the stent frame or sewing ring, and a shorter distance of the prosthetic valve leaflets from the coronary ostia attributable to the supra-annular position. In the VIVID registry, device malposition (10.3% vs. 6.2%, P = 0.014), second THV implantation (7.9% vs. 3.4%, P <0.001), and coronary obstruction (6.0% vs. 1.5%, P <0.001) occurred more frequently in stentless TAV-in-SAV. In addition, Redo TAVI is more complex than TAV-in-SAV because of the unique design of THV devices, compatibility issues, and the need for individualized planning based on factors such as implant depth, shape, and coronary artery relationships. Therefore, operators must assess these risks during a detailed preprocedural planning for each individual. Correct identification of the type and size of the implanted bioprosthesis is an important consideration when performing Valve-in-Valve TAVI. This information is usually obtained from previous SAVR/TAVI operative notes or valve identification cards provided by the manufacturers. Similarly, pre-procedural MDCT plays a key role in determining the size of THV and identifying the risk of coronary obstruction in valve-in-valve TAVI (TAV-in-SAV and TAV-in-TAV). A step-by-step assessment of the feasibility of valve-in-valve TAVI, including 1) identification of type and size of initial aortic bioprosthesis, 2) MDCT assessment, 3) selection of THV type and size, and 4) risk assessment of coronary obstruction, should be performed to obtain optimal results of valve-in-valve TAVI. Dedicated smartphone applications will guide the decision in terms of type and size of a THV for the valve-in-valve procedure (https://apps.apple.com/us/app/valve-in-valve/id655683780; https://apps.apple.com/us/app/valve-ppm/id1604258260; https://apps.apple.com/us/app/redo-tav/id6462937010), .
To achieve a better haemodynamic status after valve-in-valve TAVI, bioprosthetic valve fracture has been proposed. Bioprosthetic valve fracture is a technique in which the sewing ring of the failed surgical bioprosthesis is fractured by noncompliant, high-pressure balloon inflation with the goal of optimizing THV expansion and improving haemodynamics after TAV-in-SAV. Although several studies have reported improved bioprosthetic haemodynamics after TAV-in-SAV using bioprosthetic valve fracture, the overall clinical benefit remains uncertain. A report from the STS/ACC TVT registry, which included 2,975 patients who underwent valve-in-valve TAVI between 2020 and 2022 (bioprosthetic valve fracture was performed in 619 patients [21%]), found that attempted bioprosthetic valve fracture was associated with higher in-hospital mortality (odds ratio: 2.51; 95% CI: 1.30-4.84) and life-threatening bleeding (odds ratio: 2.55; 95% CI: 1.44-4.50) compared with no bioprosthetic valve fracture, while the benefits in bioprosthetic haemodynamics were modest (effective orifice area: 1.6 cm2 vs. 1.4 cm2, P <0.01; and mean gradient: 16.3 mmHg vs. 19.2 mmHg, P <0.01, respectively). Of note, when bioprosthetic valve fracture was stratified according to timing (before vs. after THV implantation), patients who underwent bioprosthetic valve fracture after THV implantation had a better haemodynamic status and similar in-hospital mortality to patients who did not undergo bioprosthetic valve fracture.
Depending on the index TAVI procedure and the aortic root anatomy, surgical explantation of the THV may be required for the treatment of TAVI failure. In the EXPLANT-TAVR registry including 269 patients who underwent TAVI explantation between 2009 and 2020, the most common indication for surgical intervention was endocarditis (43.1%), followed by SVD (20.1%), PVR (18.2%) and prosthesis-patient mismatch (10.8%), and more than 25% of patients were considered ineligible for redo TAVI due to unfavourable anatomy. Of note, explantation of THV is technically more demanding than native valve SAVR due to potential iatrogenic injury to the sinotubular junction, aorta, mitral valve, or membranous septum. In particular, explantation of self-expanding THV requires careful endarterectomy of the endothelialized stent frame, and THV may require detachment from the anterior leaflet of the mitral valve when implanted too low. Indeed, more than half of explantation cases required concomitant procedures as reported in previous studies, including aortic root repair/replacement and mitral valve repair/replacement, which may complicate the operation and perioperative management, . The perioperative risk of TAVI explantation is substantial, with reported 30-day mortality rates ranging from 10% to 30%, which is significantly higher compared with redo TAVI. Lack of procedural expertise may contribute to adverse outcomes; in the STS database from 2011 to 2018, the median number of TAVI explantations per surgeon and per institution was 1, and the mean annual volume of aortic root replacements performed at cardiac surgery centres in the US was 5.
As younger patients have a life-expectancy that may exceed the durability of the bioprosthesis, it becomes increasingly important to anticipate lifetime AVR strategies beyond the 10-15 year timeframe after the index procedure, . Given the risks associated with multiple open-heart surgeries and challenges of valve-in-valve procedures (e.g., coronary obstruction and suboptimal bioprosthetic haemodynamics), , incorporating both SAVR and TAVI in a sequence of lifetime interventions is critical (Figure 31). A SAVR-first strategy with a mechanical prosthesis or a Ross procedure is the most common scenario for young patients at present (<50 years of age). In patients at low surgical, who received a bioprosthesis at young age and require a second intervention when they are in their 60ies, redo SAVR followed by valve-in-valve TAVI as a third intervention in their 70s to 80s may be a plausible treatment strategy (SAVR–SAVR–TAVI). SAVR-TAVI-TAVI is a potentially less invasive strategy with the need for only one open-heart surgery in a lifetime. In this scenario, every effort should be made to implant the largest possible surgical bioprosthesis with/without aortic root enlargement at the index SAVR to optimise haemodynamics after anticipated future valve-in-valve TAVI. A TAVI-first strategies (TAVI-SAVR-TAVI) is another potentially less invasive strategy. This strategy may be beneficial in younger patients who are working, exercising, or wishing to become pregnant, in terms of faster recovery and no need for long-term anticoagulation. If the first THV degenerates, SAVR as a second intervention can be performed on a naive chest. A TAVI only strategy (TAVI-TAVI-TAVI) might be possible in patients with large aortic root anatomy allowing for the treatment with repeated short-frame THV Of note, all these scenarios are hypothetical considerations lacking validation. Current guidelines recommend that valve-in-valve TAVI for the treatment of degenerated bioprostheses should be considered only at a Comprehensive Valve Center (Class 2a Level of Evidence B-NR recommendation).
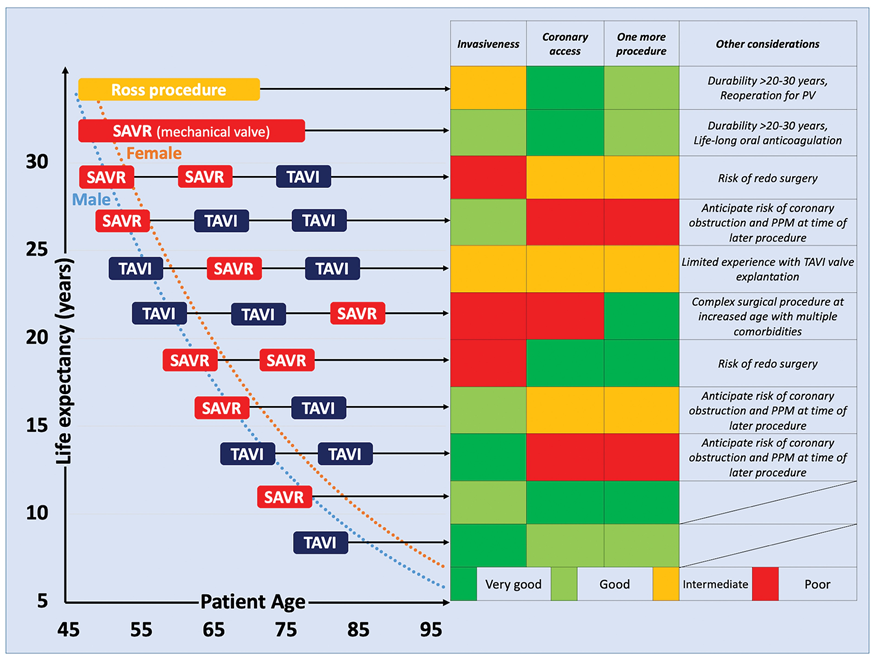
Strategies for lifetime management according to patient’s life expectancy.
Figures reproduced from Windecker et al. Eur Heart J 2022;43(29):2729-2750.
Current guidelines recommend clinical and echocardiographic follow-up for patients with asymptomatic severe AS in the absence of reduced left ventricular ejection fraction (LVEF), very severe stenosis, positive stress test, elevated biomarkers, pulmonary hypertension, rapid progression or other indications for AVR (Table 3), . However, it remains challenging to distinguish between asymptomatic and symptomatic status because symptoms are subjective and can develop insidiously, and patients adapt to symptoms, leading to underestimation of symptoms by both patients and clinicians. Observational studies, , and 2 RCTs comparing SAVR with watchful waiting (RECOVERY and AVATAR), indicate benefits of early SAVR in asymptomatic patients with severe AS. Several recent RCTs have evaluated the safety and efficacy of early intervention in patients with asymptomatic severe AS (Table 18 and Table 19).
Table 18. Ongoing randomized clinical trials of early intervention in patients with aortic stenosis.
|
Clinical Trial |
N |
Population |
Intervention |
Primary outcome |
Follow-up period |
Estimated Completion |
|---|---|---|---|---|---|---|
|
Asymptomatic severe AS |
||||||
|
ESTIMATE |
360 |
Asymptomatic severe AS patients with normal exercise test, LVEF >50%, and low surgical risk |
SAVR |
All-cause mortality and cardiac morbidity+ |
1 year |
Halted |
|
EASY-AS |
2844 |
Asymptomatic severe AS patients with LVEF ≥50% |
SAVR/TAVI |
Cardiovascular death and heart failure hospitalization |
3 years |
2029 |
|
DANAVR |
1700 |
Asymptomatic severe AS and signs of increased left ventricular filling pressure or reduced longitudinal strain |
SAVR/TAVI |
All-cause death |
5 years |
2029 |
|
Moderate AS |
||||||
|
PROGRESS |
750 |
Moderate AS with symptoms or evidence of cardiac damage/dysfunction |
TAVI |
Death, stroke, and unplanned cardiovascular hospitalization |
2 years |
2029 |
|
EXPAND TAVR II |
650 |
Moderate AS or evidence of cardiac damage/dysfunction |
TAVI |
All-cause death or unplanned procedure-related or aortic valve related hospitalization |
2 years |
2026 |
|
+: Any adverse cardiac event requiring hospitalization. Adverse cardiac events include: development of any symptom clearly related to AS (dyspnea, angina, pre-syncope or syncope during exercise); major adverse cardiac events defined as congestive heart failure or acute coronary syndrome; death of any cause, including cardiac death. AS = aortic stenosis; AVA = aortic valve area; LVEF = left ventricular ejection fraction; SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation. |
||||||
Table 19. Results of early intervention in patients with aortic stenosis.
|
Clinical Trial |
N |
Population |
Intervention |
Crossover in the clinical surveillance group |
Primary outcome |
Median F/U for primary outcome assessment |
Early intervention |
Clinical surveillance |
|---|---|---|---|---|---|---|---|---|
|
Asymptomatic severe AS |
||||||||
|
RECOVERY |
145 |
Asymptomatic very severe AS |
SAVR 100% |
74% (median 700 days) |
Operative mortality or death from cardiovascular causes |
6.2 years |
1% |
15% |
|
HR (95% CI) 0.09 (0.01-0.67) |
||||||||
|
AVATAR |
157 |
Asymptomatic severe AS and no symptoms on treadmill stress test |
SAVR 100% |
44.3% (median 476 days) |
All-cause death, acute myocardial infarction, stroke, or unplanned hospitalization for heart failure |
32 months |
15.22% |
34.7% |
|
HR (95% CI) 0.46 (0.23-0.90) |
||||||||
|
EARLY-TAVR |
901 |
Asymptomatic severe AS with age ≥65 years and no symptoms on treadmill stress test |
TAVI 100% |
87.0% (median 11.1 months) |
All-cause mortality, stroke, and unplanned cardiac hospitalization |
3.8 years |
26.8% |
45.3% |
|
HR (95% CI) 0.50 (0.40-0.63) |
||||||||
|
EVOLVED-AS |
224 |
Asymptomatic severe AS with evidence of midwall LGE |
SAVR 75% TAVI 25% |
77% (median 20.2 months) |
All-cause mortality and unplanned AS-related hospitalization |
41 months |
18% |
23% |
|
HR (95% CI) 0.79 (0.44-1.43) |
||||||||
|
Moderate AS |
||||||||
|
TAVR UNLOAD |
178 |
Moderate AS patients with reduced LVEF (<50%) and heart failure |
TAVI 100% |
43% (median 12 months) |
All-cause mortality, disabling stroke, rehospitalization, and change in KCCQ |
23 months |
47.6%* |
36.6%* |
|
Win ratio (95% CI) 1.31 (0.91-1.88) |
||||||||
|
*wins of patient pairs AS = aortic stenosis; CI = confident interval; HR = hazard ratio; KCCQ = Kansas City Cardiomyopathy Questionnaire; LVEF = left ventricular ejection fraction: LGE = late gadolinium enhancement; SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation. |
||||||||
EARLY-TAVR was a randomized, controlled trial comparing transfemoral TAVI with the SAPIEN 3 or SAPIEN 3 Ultra balloon-expandable device to clinical surveillance in patients with asymptomatic severe AS with indications for clinical surveillance. Asymptomatic status was determined by a negative treadmill stress test in 90.6% of patients. If a patient was unable to perform the stress test, eligibility was confirmed through detailed physician assessment of medical history. Between 2017 and 2021, 1,578 patients were screened and 677 (42.9%) were excluded from this trial. Among excluded patients, 313 (46.2%) were reclassified as having a class 1 indication for AVR, and the majority were confirmed to have symptomatic status by stress testing or more rigorous questioning. A total of 901 patients (mean age 76 years, 69% male, mean STS-PROM 1.8%) were randomly assigned to TAVI (N = 455) or clinical surveillance (N = 446). During a median follow-up of 3.8 years, the primary non-hierarchical composite endpoint of all-cause mortality, any stroke, or unplanned cardiovascular occurred in 122 patients (26.8%) in the TAVI group and in 202 patients (45.3%) in the clinical surveillance group (HR, 0.50; 95% CI, 0.40-0.63; P <0.001), primarily driven by guideline-directed conversions to AVR within 6 months in the clinical surveillance group. In addition, the prespecified secondary endpoint, including favourable outcome (alive and KCCQ overall score ≥75 that did not decrease by >10 points from baseline) at 2 years and the composite of integrated left ventricular health (absolute left ventricular global longitudinal strain ≥ 15%, left ventricular mass index < 115 g/m for men or < 95 g/m for women, and left atrial volume index ≤ 34 mL/m) at 2 years, was higher in the TAVI group than in the clinical surveillance group, suggesting that clinical surveillance was associated with a decline in quality of life and the development of more cardiac damage. Of note, 87.0% of patients in the clinical surveillance group underwent AVR with a median time from randomization to conversion to AVR of 11.1 months (interquartile range: 5.0 to 19.7) and with a median time to the procedure from symptom onset or from the decision to intervene of 32 days (interquartile range: 18 to 58), and there were no apparent differences in procedure-related adverse events between patients in the TAVI group and those in the clinical surveillance group who underwent AVR.
EVOLVED was a multicenter, randomized controlled trial to determine whether early AVR in asymptomatic patients with severe AS and evidence of midwall non-ischemic late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) imaging improves prognosis. The trial recruited asymptomatic patients with severe AS and preserved LVEF. At the screening visit, patients were evaluated for plasma high-sensitivity cardiac troponin I (hsTnI) concentrations and electrocardiogram. If there was evidence of left ventricular hypertrophy with strain or elevated hsTnI (an hsTnI concentration ≥6 ng/L), they proceeded to baseline CMR. A total of 224 patients with midwall LGE were enrolled and randomly allocated to the early intervention (N = 113) or the guideline-directed conservative management group (N = 111). Of 113 patients in the early intervention group, 107 (94%) underwent AVR (SAVR 75% and TAVI 25%) and 6 patients died prior to AVR during a median delay of 5.5 months. In the guideline-directed conservative management group, 85 patients (77%) underwent AVR (55% SAVR and 45% TAVI) at a median of 20.2 months. During a median follow-up of 42 months with a total follow-up of 722 patient-years, the primary composite end point of all-cause death or unplanned AS hospitalization occurred in 20 patients (18%) in the early aortic valve intervention group and 25 (23%) in the guideline-directed conservative management group (HR 0.79 [95% CI 0.44-1.43] between-group difference -4.82% [95% CI -15.31% to 5.66%]). For the secondary endpoints, early intervention was associated with a lower incidence of unplanned aortic stenosis hospitalization (6% vs. 17%, HR 0.37, 95% CI 0.16-0.88) and a lower 12-month rate of NYHA functional class II-IV symptoms than guideline-directed conservative management (19.7% vs. 37.9%; odds ratio 0.37, 95% CI 0.20-0.70). Of note, this trial was halted for 5 months due to the Covid-19 pandemic and the statistical plan was revised in light of the results of the RECOVERY and AVATAR trials.
A meta-analysis of 4 RCTs investigating the effect of an early intervention strategy in patients with asymptomatic severe AS (1,427 patients and an average follow-up time of 4.1 years) reported that early AVR was associated with a significant reduction in unplanned cardiovascular or heart failure hospitalization (pooled rate 14.6% vs. 31.9%; HR: 0.40; 95% CI: 0.30-0.53; I = 4%; P <0.01) and stroke (pooled rate 4.5% vs. 7.2%; HR: 0.62; 95% CI: 0.40-0.97; I = 0%; P = 0.03), while there were no differences in all-cause (pooled rate 9.7% vs. 13.7%; HR: 0.68; 95% CI: 0.40-1.17; I = 61%; P = 0.17) and cardiovascular mortality (pooled rate 5.1% vs. 8.3%; HR: 0.67; 95% CI: 0.35-1.29; I = 50%; P = 0.23) between early AVR and clinical surveillance .
These results suggest that early intervention is not associated with an increased risk of periprocedural complications compared with delayed intervention, improves quality of life, and reduces unplanned hospitalizations in patients with asymptomatic severe AS. Although uncertainty remains regarding valve durability, this concern does not discourage an early intervention strategy in asymptomatic patients with severe AS, given that crossover in the clinical surveillance groups occurred within 1-2 years of randomization in most patients (Table 19). Conversely, a watchful waiting strategy may be justified in the absence of an increased risk of mortality as long as careful surveillance and timely intervention can be assured in the respective health care setting.
Current guidelines recommend aortic valve intervention for moderate AS only in patients with indications for other cardiac surgery (Table 3). Observational data suggest that less than severe AS is associated with impaired prognosis, heart failure hospitalizations, and evidence of cardiac damage compared to patients without AS. In the National Echocardiographic Database of Australia, patients with moderate AS had higher 5-year all-cause and cardiovascular mortality compared with those without AS (61.4% vs. 18.6% and 43.0% vs. 10.4%, respectively). Similarly, data on 1,669,536 echocardiographic reports from 24 US hospitals reported that untreated moderate AS was associated with an increased risk of mortality compared with those without AS (adjusted HR 1.56, 95% CI 1.42-1.71). However, it has not been established whether moderate AS is an independent predictor of adverse outcomes or a surrogate marker for other prognostically relevant comorbidities. In a propensity score matched analysis of 262 pairs of patients with heart failure (LVEF <50%) alone versus heart failure and moderate AS, moderate AS was associated with an increased risk of mortality (HR 2.98; 95% CI: 2.08-4.31) and the composite of heart failure hospitalization and mortality (HR: 2.34; 95% CI: 1.72-3.21) during a median follow-up time of 2.9 years. Furthermore, the additive negative prognostic value of moderate AS is more pronounced among patients with more advanced cardiac damage. A retrospective analysis of 1,245 patients with moderate AS categorized into 5 groups according to the extent of extra-aortic valvular cardiac abnormalities demonstrated that there was a stepwise increase in all-cause mortality at 5 years according to increasing stages of cardiac damage, and advanced cardiac damage was associated with a progressively increasing risk of all-cause mortality compared with no cardiac damage (i.e., cardiac stage 0) . These observations raise the question whether aortic valve intervention for moderate AS may have a prognostic benefit, particularly in patients with impaired cardiac function.
The TAVR UNLOAD trial was the first RCT to evaluate the treatment of moderate AS in patients with reduced LVEF ranging between 20% and 50%. Conducted between January 2017 and December 2022, the trial enrolled 178 patients (mean age: 77 years; 20.8% female; 55.6% in NYHA functional class III or IV). Participants were randomized to early intervention with transfemoral TAVI using a balloon-expandable valve (N = 89) or clinical surveillance (N = 89). The primary endpoint was a hierarchical composite of: 1) all-cause mortality, 2) disabling stroke, 3) disease-related hospitalizations or heart failure equivalents, and 4) changes from baseline in KCCQ overall summary score, analysed using the win ratio. Over a median follow-up of 23 months, 38 patients (43%) in the clinical surveillance group underwent TAVI with a median crossover time of 12 months. For the primary endpoint, early TAVI resulted in wins in 47.6% of patient pairs, compared with 36.6% in the clinical surveillance group, yielding a win ratio of 1.31 (95% CI: 0.91-1.88; P = 0.14) (Table 19). At 1 year, early TAVI demonstrated superiority in the ranked ordinal comparison (48% vs. 30.9%; win ratio: 1.55; 95% CI: 1.04-2.31), primarily driven by improvements in the KCCQ overall summary score. It is noteworthy that the trial design was adapted reducing the initially planned enrolment of 600 patients to 178 due to slow recruitment and funding limitations. Moreover, while the original study design specified a primary analysis at 1 year, the extended follow-up period, combined with a 43% crossover rate in the clinical surveillance arm at a median of 12 months, may have diluted the ability to observe early clinical benefits, .
Currently, two RCTs - PROGRESS (NCT04889872) and EXPAND TAVR II (NCT05149755) - are ongoing to evaluate whether early intervention by means of TAVI improves clinical outcomes in patients with moderate AS and evidence of heart failure or cardiac damage (Table 18). These trials will determine whether earlier aortic valve intervention can improve clinical outcomes beyond the current strategy of optimal medical therapy alone.
The cumulative evidence of TAVI was obtained in well-selected patients with tricuspid aortic valve stenosis, excluding those with bicuspid aortic valve lesions. Bicuspid aortic valve is the most prevalent congenital heart condition in adults and is associated with early progression of valve deterioration, leading to the development of aortic valve disease requiring intervention in 80% of patients with bicuspid aortic valve over their lifetime, . Approximately 20% of patients undergoing SAVR aged 65-79 years and 5% of patients aged 80 years or older have bicuspid morphology, and therefore it is natural that TAVI is increasingly tested also in patients with bicuspid AS (Table 20).
Table 20. TAVI for bicuspid aortic stenosis.
| Study | N (total) |
Device | Age (years) |
Male | STS-PROM | BAV type (Siever classification) |
Post procedure ≥mod PVR |
Clinical outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up | All-cause mortality | Stroke | ||||||||
|
TAVI in bicuspid valve |
||||||||||
| Wijesinghe et al. (2010) |
11 | BEV 100% | 73.2 ± 12.5 | 55% | 4.4 ± 2.6 | NA | 27% | 30 days | 18% | NA |
| Himbert et al. (2012) |
15 | SEV 100% | 80 ± 10 | 80% | 8 ± 5 | Type 0: 13% Type I: 67% Unknown: 20% |
13.3% | 30 days | 7% | 0% |
| Mylotte et al. (2014) |
139 | BEV 34.5% SEV 65.5% |
78.0 ± 8.9 | 56.1% | 4.9 ± 3.4 | Type 0: 27% Type I: 68% Type II: 5% |
34% | 30 days | 5.0% | 2.2% |
| 1 year | 17.5% | NA | ||||||||
| Yousef et al. (2015) |
108 | BEV 56% SEV 44% |
75.5 ± 14.4 | 63.9% | 17.2 ± 12.2 | Type 0: 12% Type I: 53% Type II: 7% Unknown: 28% |
31% | 30 days | 8.3% | 2.8% |
| 1 year | 16.9% | NA | ||||||||
| Kosek et al. (2015) |
7 | BEV 29% SEV 71% |
77.7 (72–85) | 43% | NA | NA | 28% | 30 days | 14% | 0% |
| Perlman et al. (2016) | 51 | BEV 100% | 76.2 ± 9.3 | 47.1% | 5.2 ± 3.7 | Type 0: 11.8% Type I: 74.4% Type II: 1.9% Unknown: 11.6% |
0% | 30 days | 3.9% | 1.9% |
| Yoon et al. (2016) |
301 | BEV 59% SEV 37% MEV 4% |
77.0 ± 9.2 | 57.5% | 4.7 ± 5.2 | Type 0: 10.3% Type I: 74.4% Type II: 1.7% Unknown: 13.6% |
6% | 30 days | 4.3% | 2.3% |
| 1 year | 14.4% | NA | ||||||||
| Jilaihawi et al. (2016) |
130 | BEV 54% SEV 46% |
76.6 ± 10.4 | 61.5% | 4.7 (3.0-7.3) | Type 0: 20.9% Type I: 54.9% Type II: 0% Unknown: 24.2% |
18% | 30 days | 3.8% | 3.3% |
| Yoon et al. (2017) |
108 | BEV 100% | 74.4 ± 10.6 | 71.3% | 5.2 ± 3.4 | Type 0: 6% Type I: 94% |
6% | 30 days | 0.9% | 4.6% |
| 1 year | 6.9% | NA | ||||||||
| Liu et al. (2018) |
12 | SEV 100% | 76 ± 4 | 67% | 6.86 ± 4.27 | Type 0: 67% Type I: 33% |
0% | 30 days | 0% | 8% |
| Kim et al. (2018) |
144 | BEV 63% SEV 32% MEV 5% |
NA | NA | NA | Type 0: 4.2% Type I complete raphe: 41.7% Type I partial raphe: 54.1% |
11.1% | 30 days | 4.9% | NA |
| Mangieri et al. (2018) |
353 | BEV 68.6% SEV 31.4% |
77.8 ± 8.3 | 64.9% | 4.4 ± 3.3 | Type 0: 7.1% Type I: 61.8% Type II: 0.9% Unknown: 29.8% |
4.0% | 30 days | 4.3% | 1.6% |
| Attinger-Toler et al. (2019) |
79 | BEV 100% | 76 ± 9 | 56% | 3.8 (2.3–5.5) | Type 0: 6% Type I L-R: 65% Type I R-N: 14% Type I L-N: 2% Type II: 5% Unknown: 8% |
5% | 30 days | 3.8% | 1.3% |
| 1 year | 7.7% | 1.3% | ||||||||
| Lei et al. (2019) |
71 | SEV 77.5% MEV 22.5% |
71.9 ± 5.8 | 45.1% | 7.0 ± 3.6 | Type 0: 100% | 0% | 30 days | 7.0% | 4.2% |
| 1 year | 8.5% | NA | ||||||||
| Weir-McCall et al. (2019) |
44 | BEV 100% | 75 ± 10 | 59% | 4.3 ± 2.3 | Type 0: 5% Type I L-R: 77% Type I R-N: 18% Type I L-N: 0% Type II: 0% |
0% | 1 year | 8.9% | NA |
| LRT trial Bicuspid Arm (2019) |
61 | BEV 74% SEV 26% |
68.6 ± 7.4 | 42.6% | 1.5 ± 0.6 | Type 0: 13.3% Type I: 78.3% Type II: 3.3% |
2.0% | 30 days | 0% | 1.6% |
| Mangieri et al. (2020) |
353 | BEV 68.6% SEV 31.4% |
77.8 ± 8.3 | 64.9% | 4.4 ± 3.3 | Type 0: 7.1% Type I: 61.8% Type II: 0.9% Unknown: 29.8% |
4.0% | 30 days | 4.3% | 1.6% |
| Yoon et al. (2020) |
1034 | BEV 71.6% SEV 23.9% MEV 4.5% |
74.7 ± 9.3 | 59% | 3.7 ± 3.3 | Type 0: 10.3% Type I: 89.7% |
5.2% (≥Mild-moderate) | 30 days | 2.0% | NA |
| 2 years | 12.5% | 2.7% | ||||||||
| Kochman et al. (2020) |
24 | MEV 100% | 75.3 ± 7.85 | 50% | 13.4 ± 10.39* | Type 0: 8% Type I: 75% Type II: 0% Functional: 8% Unknown: 8% |
4% | 30 days | 4% | 4% |
| 2 years | 12.5% | 4.2% | ||||||||
| Attinger-Toller et al. (2020) |
79 | BEV 100% | 76 ± 9 | 56% | 3.8 (2.3–5.5) | Type 0: 6% Type I: 81% Type II: 5% Unknown: 8% |
0% | 30 days | 3.8% | 1.3% |
| 1 year | 7.7% | 1.3% | ||||||||
| Gorla et al. (2021) |
56 | SEV 73.2% MEV 26.8% |
75.5 ± 12.4 | 62% | 3.4 ± 2.1 | NA | 14% | 30 days | 4% | 2% |
| Ielasi et al. (2021) |
243 | BEV 70% SEV 30% |
80 ± 7.9 | 67.1% | 3.5 ± 4.6 | Type 0: 10.3% Type I: 89.7% |
4.1% | 30 days | 4% | 1.4% |
| 1 year | 9.8% | 7.3% | ||||||||
| Kumar et al. (2022) |
70 | BEV 30% SEV 70% |
72 ± 8.49 | 68.6% | 6.00 ± 6.54 | Type 0: 21% Type I: 78% |
0% | 30 days | 2.86% | 0% |
| 2 year | 2.86% | 0% | ||||||||
| Elkoumy et al. (2022, 2023) |
68 | BEV 100% | 72.6 ± 9.4 | 72% | 3.54 ± 2.1 | Type 0: 19% Type I: 74% Type II: 7% |
3% | 30 days | 3.0% | 0% |
| 1 year | 11.3% | 3.2% | ||||||||
| Tchétché et al. (2023) |
149 | SEV 100% | 78 | 63.1% | 2.6 (1.7-4.2) | Type 0: 10.1% Type I: 72.5% Type II: 3.3% |
11.3% (≥Mild-moderate) | 1 year | 10% | 4% |
| Li et al. (2023) |
156 | SEV 100% | 71.6 ± 6.8 | 64.7% | 2.1 (1.5–3.7) | NA | 6.0% | 30 days | 2.6% | 0.6% |
| Deutsch et al. (2023) |
106 | BEV 64% SEV 36% |
NA | 65% | 2.6 | Type 0: 7.5% Type I: 92.5% Type II: 0% |
0% | 30 days | 0.9% | 2.8% |
| Fiorina et al. (2023) |
109 | BEV 31.2% SEV 56% MEV 12.8% |
78 ± 7.5 | 49.5% | 5.1 ± 4.3 | NA | 13.1% | 4 years | 32% | NA |
| Boiago et al. (2023) |
150 | BEV 55.3% SEV 44.7% |
81.4 ± 7.5 | 67.3% | 5.4 ± 6.4 | Type 0: 6.7% Type I: 92.7% Type II: 0.7% |
9.9% | 30 days | 3.4% | 2% |
| 3 years | 28.1% | 9.6% | ||||||||
| Evolut Low Risk Bicuspid Study (2020, 2021, 2024) |
150 | SEV 100% | 70.3±5.5 | 52.0% | 1.3 (0.9, 1.7) | Type 0 9.3% Type 1 90.7% Type 2 0% |
0% | 30 days (N = 50) | 0.7% | 4.0% |
| 1 year | 0.7% | 4.7% | ||||||||
| 2 years | 2.1% | 6.1% | ||||||||
| 3 years | 2.8% | 6.9% | ||||||||
|
TAVI in bicuspid vs. tricuspid valves |
||||||||||
| Hayashida et al. (2013) |
21 (229) |
BEV 52% SEV 48% |
82.0 ± 7.0 | 57.1% | NA | Type I: 86% Type II: 14% |
19% | 30 days | 7.9% | NA |
| Bauer et al. (2014) |
38 (1395) |
BEV 32% SEV 68% |
80.7 ± 6.6 | 45% | 18 ± 10* | NA | 25% | 30 days | 11% | 0% |
| 1 year | 13% | 0% | ||||||||
| Costopoulos et al. (2014) |
21 (468) |
BEV 38% SEV 62% |
76.7 ± 7.1 | 57% | 7.6 ± 4.2 | NA | 24% | 30 days | 14% | 0% |
| 1 year | 32% | NA | ||||||||
| Kochman et al. (2014) |
28 (112) |
BEV 15% SEV 85% |
77.6 ± 5.5 | 46% | 19.2 ± 9.0* | NA | 32% | 30 days | 4% | 0% |
| 1 year | 18% | NA | ||||||||
| Liu et al. (2015) |
15 (40) |
SEV 100% | 75.4 ± 5.7 | 60% | 5.6 ± 4.1 | Type 0: 73% Type I: 27% Type II: 0% |
0% | 30 days | 6.7% | 6.7% |
| Yoon et al.† (2017) |
546 (1092) |
BEV 58% SEV 34% MEV 8% |
77.2 ± 8.2 | 62.8% | 4.6 ± 4.6 | Type 0: 11% Type I: 75% Type II: 1% Unknown: 13% |
10% | 30 days | 3.7% | 2.9% |
| Arai et al. (2017) |
10 (153) |
BEV 100% | 81.3 ± 5.1 | 43% | 19.0 ± 12.5* | Type I: 90% Type II: 10% |
0% | 30 days | 0% | 0% |
| Sannino et al. (2017) |
88 (823) |
BEV 52% SEV 48% |
80.2 ± 8.4 | 60.2% | 7.4 ± 3.9 | Type 0: 14% Type I: 85% Type II: 1% |
5% | 30 days | 3.4% | 2.3% |
| 1 year | 8.5% | NA | ||||||||
| Song et al. (2018) |
44 (97) | SEV 100% | 73.8 ± 5.2 | 54.5% | 5.0 (3.8–8.2) | Type 0: 50% Type I complete raphe: 45% Type I partial raphe: 5% |
11.4% | 30 days | 6.8% | 2.3% |
| 2 years | 11.4% | NA | ||||||||
| Aalaei-Andabili et al. (2018) |
32 (128) |
BEV 78% SEV 22% |
68.59 ± 11.07 | 66.7% | 6.01 ± 3.42 | Type 0: 75% Type I: 12.5% Type II: 6.25% Unknown: 6.25% |
3% | 30 days | 6.25% | 6.2% |
| 2 years | 14% | 6.2% | ||||||||
| Liao et al. (2018) |
87 (157) |
SEV 100% | 73.4 ± 6.4 | 57.5% | 7.9 ± 4.0 | Type 0: 56% Type I: 44% |
14% | 30 days | 9.2% | 1.1% |
| Nagaraja et al.† (2019) |
359 (718) |
NA | 68.01 ± 13.42 | 34.83% | NA | NA | NA | In-hospital | 5.57% | 2.79% |
| Tchetche et al. (2019) |
101 (189) |
BEV 65.3% SEV 28.8% MEV 9.9% |
78.2 ± 10.1 | 65% | 11.3 ± 8.5 | Type 0: 12.9% Type I: 86.1% Type II: 1% |
20.8% (Mild-moderate) | 30 days | 0% | 2% |
| Makkar et al.† (2019) |
2691 (5382) |
BEV 100% | 74 (66-81) | 60.3% | 4.9 ± 4.0 | NA | 2.0% | 30 days | 2.6% | 2.5% |
| 1 year | 10.5% | 3.4% | ||||||||
| LRT trial Bicuspid Arm† (2019) |
50 (100) |
BEV 74% SEV 26% |
68.6 ± 7.4 | 42.6% | 1.5 ± 0.6 | Type 0: 13.3% Type I: 78.3% Type II: 3.3% |
2.0% | 30 days | 0% | 1.6% |
| Harim et al. (2020) |
5412 (170959) |
BEV 81.1% SEV 18.9% |
74.0 (65.0–81.0) | 59.1% | 3.8 (2.3–6.1) | NA | 4.4% | In-hospital | 2.0% | 2.2% |
| Forrest et al.† (2020) |
929 (1858) |
SEV 100% | 73.0 ± 10.3 | 55.1% | 5.3 ± 4.2 | NA | 2.2% | 30 days | 2.6% | 3.4% |
| 1 year | 10.4% | 3.9% | ||||||||
| Pineda et al. (2020) |
50 (567) |
BEV 38% SEV 62% |
70 (64-74) | 64% | 4.6 (3.0-7.7) | Type 0: 14.0% Type I: 86.0% |
4.0% | 30 days | 6.0% | 2.0% |
| 2 years | 18.0% | NA | ||||||||
| Fu et al. (2020) |
44 (118) |
SEV 100% | 73.57 ± 6.30 | 52.3% | 6.70 ± 2.95 | NA | 11.4% | 30 days | 4.5% | 2.3% |
| 1 year | 13.6% | 6.8% | ||||||||
| Chodór et al. (2021) |
21 (83) |
SEV 100% | 75.76 ±7.96 | 66.67% | 21.63 ±11.49 | NA | 14.28% | 30 days | 4.76% | 0% |
| 1 year | 28.57% | 0% | ||||||||
| Makkar et al.† (2021) |
3168 (6336) |
BEV 100% | 68.8 ± 8.7 | 69.2% | 1.7 ± 0.6 | NA | 1.8% | 30 days | 0.9% | 1.4% |
| 1 year | 4.6% | 2.0% | ||||||||
| Michel et al. (2021) |
78 (743) |
BEV 100% | 77 , | 61.5% | 9.12 [5.84, 14.72] | Type 0: 8.9% Type I: 91% |
1.3% | 30 days | 0% | 3.8% |
| 1 year | 1.3% | 3.8% | ||||||||
| Gasecka et al.† (2022) |
130 (520) |
BEV 34% SEV 66% |
80 (76–84) | 51% | 3.8% (2.8–6.5%)* | NA | 2% | In-hospital | 2.1% | 2% |
| 10 years | 22.5% | NA | ||||||||
| Zhou et al. (2022) |
109 (246) |
BEV 4.6% SEV 83.5% MEV 11.9% |
75 (71-80) | 56.9% | 5.09 (3.65-8.62) | Type 0: 61.5% Type I: 36.7% Type II: 1.8% |
6.5% | 3 years | 12.8% | 7.3% |
| Ullah et al.† (2022) |
2673 (5347) |
NA | 67.91 ± 14.2 | 62.4% | NA | NA | NA | 30 days | NA | NA |
| 6 mohts | NA | NA | ||||||||
| PARTNER 3 Bicuspid Registry† (2022) |
148 (296) |
BEV 100% | 71 (68-75) | 58.1% | 1.4 (1.0, 1.9) | Type 0: 13.6% Type I: 85.8% Type II: 0.6% |
1.4% | 30 days | 0% | 1.4% |
| 1 year | 0.7% | 2.1% | ||||||||
| Dai et al. (2023) |
211 (402) |
SEV 100% | 74.0 (69.0–79.0) | 58.3% | 4.4 (2.6–7.6) | NA | NA | 30 days | 2.4% | NA |
| 1 year | 5.2% | NA | ||||||||
| Hong et al. (2023) |
229 (389) |
SEV 100% | 72.9 ± 6.9 | 65.1% | 2.6 ± 0.9 | NA | 2.6% | 30 days | 0.9% | 3.3% |
| Mukai et al. (2023) |
423 (17225) |
BEV 56.5% SEV 43.5% |
<65 yrs 1.9% <75 yrs 11.8% <85 yrs 47.5% ≥85 yrs 47.5% |
53.2% | 5.4 (3.5–8.2) | NA | 3.2% | 30 days | NA | 1.9% |
| 1 year | 7.9% | NA | ||||||||
| He et al.† (2023) |
242 (363) |
Type 0: BEV 5.2%, SE 94.8% Type I: BEV 8.9%, SEV 91.1% |
Type 0: 73.04 ± 5.97 Type I: 72.51 ± 8.03 |
Type 0: 62.8% Type I: 62.8% |
Type 0: 5.16 ± 3.94 Type I: 4.97 ± 4.41 |
NA | Type 0: 13.2% Type I: 14.5% |
30 days | Type 0: 4.2% Type I: 1.7% |
Type 0: 1.0% Type I: 0.9% |
| 1 year | Type 0: 10.0% Type I: 2.3% |
Type 0: 1.4% Type I: 1.6% |
||||||||
| Magyari et al.† (2023) |
52 (104) |
BEV 100% | 71 ± 7.1 | 65.4% | 5.2 ± 3.3 | NA | NA | 30 days | 0% | 1.9% |
|
TAVI vs. SAVR in bicuspid valve |
||||||||||
| Elbadawi et al.† (2019) |
975 (1950) |
NA | 65.70 ± 16.53 | 60% | NA | NA | NA | In-hospital | 3.1% | 2.1% |
| Soud et al.† (2020) |
68 (136) | NA | 65.0 ± 14.8 | 67.6% | NA | NA | NA | In-hospital | 5.9% | NA |
| Mentias et al.† (2020) |
699 (1398) |
NA | 74.7 ± 9.4+ | NA | NA | NA | NA | 30 days | 2.9% | 4.0% |
| 1 year | 9.0% | 4.0% | ||||||||
| Tsai et al. (2021) |
48 (130) |
NA | 65.7 ± 7.8 | 58.3% | 6.25 ± 6.4 | NA | NA | In-hospital | 12.5% | 2% |
| Majmundar et al.† (2022) |
1393 (2786) |
NA | 68.3 ± 10.1 | 62.2% | NA | NA | NA | 30 days | 0.3% | 0.1% |
| 6 months | 0.4% | 0.1% | ||||||||
| Sanaiha et al.† (2023) |
3855 (56331) |
NA | 69 (62-76) | 61.2% | NA | NA | NA | In-hospital | 1.6% | 0.8% |
| Chen et al.† (2023) |
797 (1594) |
NA | 73 (69-77) | 58.2% | NA | NA | NA | 3 years | 25.4% | NA |
| NOTION-2 Bicuspid cohort (2024) |
49 (100) |
BEV 28.5% SEV 67.3% MEV 4.1% |
69.7 ± 3.6 | 55.1% | 1.0 (0.8-1.3) | Type 0: 6.1% Type I: 91.8% Type II: 2.1% |
9.1% (at 1 year) |
1 year | 4.1% | 6.1% |
|
Post procedural PVR was evaluated at discharge or 30 days. †Matched cohort. +Entire cohort. *EuroSCORE. BEV = balloon expandable valve; MEV = mechanically-expanding valve; PVR = paravalvular regurgitation; SAVR = surgical aortic valve replacement; SEV = self-expanding valve; STS-PROM = Society of Thoracic Surgeons Predicted Risk of Mortality; TAVI = transcatheter aortic valve implantation. |
||||||||||
Bicuspid valve morphology may pose a technical challenge for TAVI due to anatomical specifications, including a non-circular aortic root, more severe and asymmetric distribution of leaflet calcification, presence of 1 or 2 raphes with variable degree of calcification and associated aortopathy (Figure 32), . Indeed, early experience with TAVI in patients with severe bicuspid AS has highlighted an increased risk of procedural complications and lower device success compared with TAVI in patients with tricuspid AS. Device advances, increasing operator experience, and careful patient selection based on the systematic use of preprocedural MDCT for the assessment of aortic valve and root morphology have improved procedural success and device performance with TAVI in patients with bicuspid AS. The STS/ACC TVT registry reported comparable rates of device success, bioprosthetic valve haemodynamics, and 1-year clinical outcomes using newer-generation devices (balloon-expandable SAPIEN 3 and self-expanding Evolut R or PRO devices) in selected patients with bicuspid compared with tricuspid AS, . However, even with TAVI using newer-generation devices, bicuspid morphology remained associated with a higher risk of peri-procedural stroke and significant PVR, . Furthermore, a core laboratory MDCT analysis in a multinational registry of 1,034 bicuspid AS patients undergoing TAVI, identified calcified raphe and excess leaflet calcification in bicuspid aortic valve as the risk factors for procedural complications and mid-term mortality after TAVI using current-generation devices.
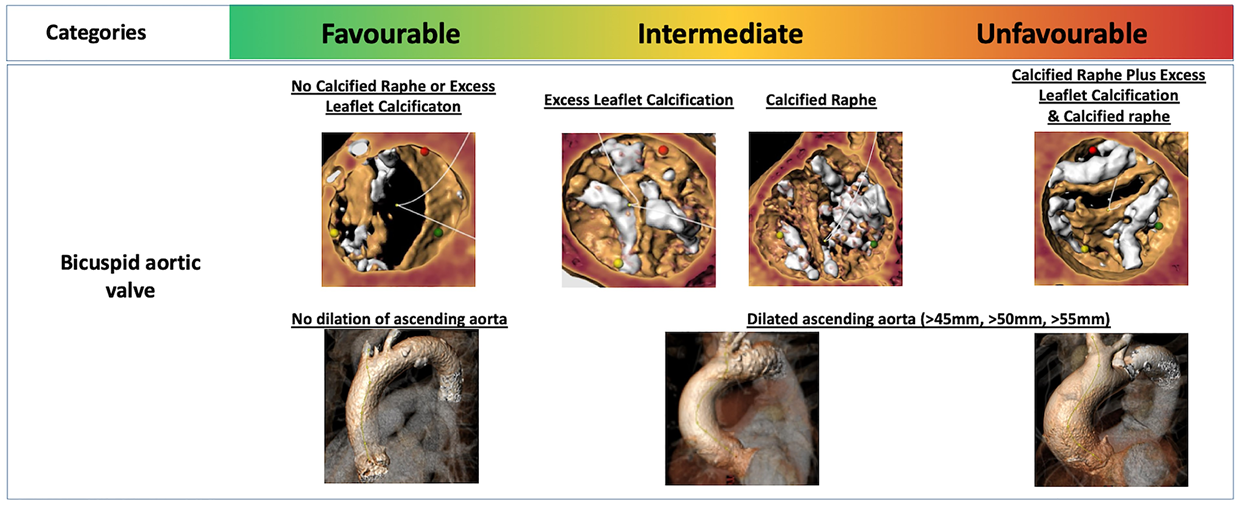
Anatomical risk stratification of bicuspid aortic valve.
Figures reproduced from Windecker et al. Eur Heart J 2022;43(29):2729-2750.
Consistent with TAVI for tricuspid AS, evidence has accumulated to support the feasibility of TAVI in low-risk patients with bicuspid AS. The STS/ACC TVT registry provided data from a propensity score-matched analysis of 3,168 bicuspid and tricuspid AS pairs undergoing TAVI with the SAPIEN 3 balloon-expandable device (mean age 69 years; 69.8% men; mean STS-PROM 1.7%) and reported no significant differences in procedural complications, bioprosthetic performance, and clinical outcomes at 30 days and 1 year. Similar findings have been reported in the bicuspid registries from the pivotal low-risk randomized clinical trials. In the PARTNER 3 bicuspid registry (N = 148, mean age 71 years; 58.1% men; mean STS-PROM 1.4%), patients with bicuspid AS treated with SAPIEN 3 balloon-expandable device had similar procedural success (100% vs. 99.3%) and 1-year clinical outcomes (death: 0.7% vs. 1.4%; stroke: 2.1% vs. 2.0%; cardiovascular rehospitalization: 9.6% vs. 9.5%) compared to patients with tricuspid AS in a propensity score matching analysis. The Evolut Low Risk Bicuspid Study provides the longest-term prospective follow-up data on 150 low-risk patients with bicuspid AS undergoing TAVI with the self-expanding Evolut R or PRO device (mean age: 70.3 years, median STS-PROM: 1.3%). Periprocedural outcomes were favourable with device success rate of 95.3% and no ≥moderate PVR, and 30-day mortality and disabling stroke were rare (both occurred in 1 patient). A propensity-score matched comparison of this group with the TAVR cohort of the Evolut Low Risk randomized trial reported no relevant differences in clinical and bioprosthetic haemodynamics at 1 year between the bicuspid and tricuspid anatomies. At 3 years, the composite of all-cause death or disabling stroke, all-cause death, disabling stroke, and aortic valve hospitalization occurred in 4.1%, 2.8%, 2.1%, and 5.4% of patients, respectively, and bioprosthetic haemodynamic properties were sustained with 15% mild PVR and no moderate or greater PVR. These data suggest that TAVI is feasible in younger, low-risk patients with bicuspid aortic valve morphology taking into consideration that the low-risk bicuspid populations were highly selected with strict anatomical exclusion criteria for relevant device landing zone calcification evaluated by a review committee, as well as the exclusion of patients with concomitant relevant valvular or coronary artery disease, , .
Several uncertainties remain in patients with bicuspid AS who are candidates for TAVI: 1) definition of favourable and unfavourable bicuspid aortic valve morphology; 2) optimal device selection and sizing strategy; 3) long-term TAVI durability and evolution of aortopathy after TAVI. Current clinical guidelines recommend SAVR as the preferred treatment strategy in patients with bicuspid aortic valve morphology (Table 21).
Table 21. Guideline recommendations: Mode of intervention in patients with bicuspid aortic stenosis.
|
2020 AHA/ACC Valvular Heart Disease Guideline |
2021 ESC/EACTS Valvular Heart Disease Guideline |
||||
|---|---|---|---|---|---|
|
COR |
LOE |
Recommendation |
COR |
LOE |
|
|
2b |
B-NR |
In patients with bicuspid aortic valve and symptomatic, severe AS, TAVI may be considered as an alternative to SAVR after consideration of patient-specific procedural risks, values, trade-offs, and preferences, and when the surgery is performed at a Comprehensive Valve Center. |
Bicuspid aortic valve is an anatomical factor that favors SAVR. |
NA |
|
|
SAVR remains more appropriate in patients with AS affecting a bicuspid valve and in those with associated disease (e.g. aortic root dilatation, complex coronary disease, or severe mitral regurgitation) requiring a surgical approach. |
NA |
||||
|
ACC/AHA = American College of Cardiology/American Heart Association; AS = aortic stenosis; COR = class of recommendation; ESC/EACTS = European Society of Cardiology and the European Association for Cardio-Thoracic Surgery; LOE = Level of evidence; SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation. |
|||||
The available evidence on the safety and efficacy of TAVI comparing SAVR is mainly limited to observational studies, , . The NOTION-2 trial is the only randomized trial comparing TAVR and SAVR in patients aged ≤75 years at low surgical risk that did not exclude patients with bicuspid aortic valve morphology. The bicuspid substudy comprised 100 patients (49 TAVI and 51 SAVR), and patients in the bicuspid arm were younger and less likely to have comorbidities compared to patients in the tricuspid arm. At 1 year, the primary endpoint (death from any cause, stroke, or rehospitalization) was higher in the TAVI group than in the SAVR group (14.3% vs. 3.9%), primarily driven by higher rates of stroke (6.1% vs. 0%). Although the sample size of the bicuspid cohort in the NOTION-2 was small and underpowered to determine the optimal strategy in this population, these data suggest that the results of TAVI in tricuspid AS may not be generalizable to patients with bicuspid AS. Currently, dedicated randomized trials comparing TAVI with SAVR in this specific setting with long-term follow-up are in the preparation phase. These trials will provide evidence-based data to inform future guideline recommendations. In the meantime, Heart Teams need to provide a tailored approach for individual patients with bicuspid AS based on the patient’s risk profile, comorbidities, anatomical considerations, and life expectancy.
Patients with severe AS may present with acute decompensated heart failure or cardiogenic shock, a critical clinical status with poor short-term prognosis. Although SAVR has been considered in these patients, the surgical approach has been associated with an increased risk of perioperative mortality in the emergent and/or acute setting. As a result, TAVI has gained increasing attention in the emergent/urgent setting (emergent/urgent TAVI) as a viable therapeutic option among patients with acute decompensated heart failure or cardiogenic shock. Results of patients undergoing emergent TAVI have been consistent, indicating technical feasibility but an increased risk of short-term mortality, , , , , , , . A recent analysis from the STS/ACC TVT registry including 309,505 patients undergoing TAVI with a balloon-expandable device reported that 5,006 patients (1.6%) presented with cardiogenic shock (defined according to registry coding data, pre-procedural use of inotropic or mechanical circulatory support, and/or cardiac arrest within 24 hours prior to the procedure) prior to TAVI and that VARC-3 technical success rate was 95%. In a propensity-matched analysis, TAVI in the setting of cardiogenic shock was associated with an increased risk of in-hospital (9.9% vs. 2.7%), 30-day (12.9% vs. 4.9%), and 1-year (29.7% vs. 22.6%) mortality compared with the control cohort. Using landmark analyses, the adverse impact of emergent TAVI associated with the critically ill clinical status was limited to the first 30 days after TAVI, whereas there were no significant differences between groups during the time period between 30 days and 1 year of follow-up. In addition, there was significant improvement in functional status among patients surviving after the procedure. These results suggest that patients with severe AS who are critically ill due to acute decompensation or cardiogenic shock are potential candidates for emergent TAVI in the absence of severe comorbidities.
Aortic regurgitation (AR) is another aortic valve disease entity associated with relevant morbidity and mortality. In the OxVALVE population cohort study of 2,500 individuals aged ≥65 years representing a primary care population, the prevalence of any and moderate or severe AR was 15% and 1.6%, respectively. Patients with significant AR are asymptomatic for a long period of time, although AR results in severe left ventricular volume and pressure overload, leading to detrimental structural and functional remodeling associated with poor survival, . Surgical aortic valve repair or replacement is the standard of care for patients with severe symptomatic AR, and current guidelines limit the use of TAVI for the treatment of pure AR to patients who are ineligible for surgery (Table 22), .
Table 22. Guideline recommendations: Mode of intervention in patients with pure aortic regurgitation
|
2020 AHA/ACC Valvular Heart Disease Guideline |
2021 ESC/EACTS Valvular Heart Disease Guideline |
|---|---|
|
Recommendation |
|
|
TAVI for isolated chronic AR is challenging because of dilation of the aortic annulus and aortic root and, in many patients, lack of sufficient leaflet calcification. Risks of TAVI for treatment of AR include transcatheter valve migration and significant paravalvular regurgitation. TAVI is rarely feasible, and then only in carefully selected patients with severe AR and heart failure who have a prohibitive surgical risk and in whom valvular calcification and annular size are appropriate for a transcatheter approach. |
TAVI may be considered in experienced centres for selected patients with AR and ineligible for SAVR. |
|
ACC/AHA = American College of Cardiology/American Heart Association; AR = aortic regurgitation; COR = class of recommendation; ESC/EACTS = European Society of Cardiology and the European Association for Cardio-Thoracic Surgery; LOE = Level of evidence; SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation. |
|
However, there is a non-negligible undertreatment of patients with severe symptomatic AR, and the predominant reasons include advanced age, female sex, high surgical risk, and advanced left ventricular systolic dysfunction, , . Accordingly, TAVI for pure AR has been explored as an alternative treatment strategy (Table 23) although it is technically challenging due to anatomical and device-related considerations; dilatation of the ascending aorta and aortic annulus, lack of calcification of the aortic valve leaflets, elliptical and dilated LVOT, increased stroke volume, and lack of large THV sizes and anchoring capability.
Table 23. TAVI for pure native valve aortic regurgitation.
|
Study |
N |
Age (years) |
STS-PROM |
Access |
Device |
Procedural success |
Post procedure ≥mod PVR |
Second Valve Required |
30 days mortality |
1 year (6 months) mortality |
|---|---|---|---|---|---|---|---|---|---|---|
|
Roy et al. (2013) |
43 |
75.3 ± 8.8 |
10.2 ± 5.3% |
Femoral (81.4%) |
CoreValve
|
74%* |
4.7% |
18.6% |
9.3% |
21.4% |
|
Seiffert et al. (2014) |
31 |
73.8 ± 9.1 |
5.4 ± 3.6% |
Apical |
JenaValve |
96.8%* |
0% |
3.2% |
12.9% |
19.3% |
|
Wendt et al. (2014) |
8 |
72.5 ± 8.4 |
7.3 ± 3.3% |
Apical |
ACURATE TA |
100%+ |
0% |
0 |
0 |
0 |
|
Testa et al. (2014) |
26 |
73 ± 10 |
13.1 ± 2.0% |
Femoral |
CoreValve
|
76.9%+ |
88.5%a |
19.2% |
23% |
31% |
|
Schofer et al. (2015) |
11 |
74.7 ± 12.9 |
8.84 ± 8.9% |
Femoral |
Direct Flow |
100%+ |
0% |
0 |
9% |
/ |
|
Sawaya et al. (2017) |
78 |
74 ± 10 |
6.7 ± 4.8% |
Femoral (65%) |
CoreValve (42%) |
72%+ |
14% |
16.7% |
14% |
/ |
|
Yoon et al.(2017) |
331 |
74.4 ± 12.2 |
6.7 ± 6.7% |
Femoral (70.4%) |
Early generation THV#1 (35.9%) Newer generation THV#2 (64.1%) |
74.3% (Early generation THV 61.3%; Newer generation THV 81.1%)+ |
9.6% (Early generation THV 18.8%; Newer generation THV 4.2%)
|
16.6% (Early generation THV 24.4%; Newer generation THV 12.7%) |
10.9% (Early generation THV 13.4%; Newer generation THV 9.4%) |
/ |
|
De Backer et al.(2018) |
254 |
74 ± 12 |
6.6 ± 6.2% |
Femoral (76%) |
Early generation THV#1£(43.0%) Newer generation THV#2 |
67% (Early generation THV 47%; Newer generation THV 82%)+ |
15.5% (Early generation THV 31.0%; Newer generation THV 4.0%) |
NA |
11.7% (Early generation THV 17.0%; Newer generation THV 8.0%) |
37.8% |
|
STS/ACC TVT Registry |
230 |
68.7 ± 15.1 |
8.6 ± 9.1% |
/ |
CoreValve (35.2%)
|
81.7% (CoreValve 72.2%; Evolut R 86.9%)* |
10.5% (CoreValve 21.5%; Evolut R 5.2%) |
NA |
13.3% (CoreValve 19.0%; Evolut R 10.0%) |
22.8% |
|
Purita et al.(2020) |
24 |
79.4 (50-88) |
3.9 ± 2.37% |
Femoral |
ACURATE neo |
87.5%+ |
4.1% |
12.5% |
4.1% |
11.7% |
|
Liu et al. |
137 |
73.1 ± 6.4 |
9.8 ± 5.3 |
Apical (100%) |
J-Valve (100%) |
NA |
0.7% |
NA |
3% |
3.7% |
|
Lu et al. |
27 |
70.6 ± 7.1 |
16.8 ± 9.5% b |
Apical (100%) |
J-Valve (100%) |
96.3%+ |
0% |
0% |
3.7% |
3.7% |
|
Schneeberger et al. |
9 |
74.4 ± 7.1 |
6.2 ± 3.0% |
Femoral |
Acurate neo (11.1%) |
100%+ |
0% |
0% |
0% |
NA |
|
Liu et al. |
137 |
73.1 ± 6.4 |
9.8 ± 5.3 |
NA |
J-Valve (100%) |
97.1%+ |
0.7% |
0% |
0% |
7.4% |
|
Delhomme et al. |
37 |
81 (69–85) |
4.82 (2.46–6.86)b |
Femoral (94.6%) Other (5.4%) |
SAPIEN 3 (100%) |
94.6%+ |
2.7% |
0% |
8.1% |
16.2% |
|
Zeng et al.(2023) |
45 |
73.5 ± 5.5 |
28.5 ± 7.3b |
Femoral |
Venus A-Valve (100%) |
97.8%+ |
0% |
2.3% |
2.3% |
4.7% |
|
Kodali et al.(2023) |
13 |
73.9±6.4 |
2.3±1.1% |
Femoral (54%) |
DurAVR (100%) |
100%† |
0% |
0% |
0% |
0% |
|
Garcia et al. (2023) |
27 |
81 (72-85) |
NA |
Femoral (78%) |
J-Valve (100%) |
81%† |
11.1% |
0% |
4% |
12% |
|
Adam et al.(2023) |
58 |
76.5 ± 9.0 |
4.2 ± 4.3% |
Femoral |
JenaValve Trilogy (100%) |
100%† |
0% |
0% |
0% |
NA |
|
Poletti et al.(2023) |
201 |
79 (73-83) |
5.1 (4.1-6.1) |
Femoral (95.5%) |
BEV 34% |
83.6%† |
9.5% |
10.5% |
5.0% |
NA |
|
ALIGN-AR |
180 |
75.5 ± 10.8 |
4.1 ± 3.4% |
Femoral |
JenaValve Trilogy (100%) |
95%† |
<1% |
1.1% |
2% |
7% |
|
Orzalkiewicz et al. |
13 |
80.8 ± 5.6 |
4.0 ± 1.7% |
Femoral |
SAPIEN 3 (69.2%) |
92.3%† |
0% |
0% |
7.7% |
12.6% |
|
Poletti et al.(2024) |
256 |
80 (73-84) |
3.1 (2.1-5.0) |
Femoral |
JenaValve Trilogy (34%) |
98%† |
1.1% |
1.1% |
1.1% |
17.2% |
|
Off-label THV (66%) |
81%† |
10% |
11% |
6.6% |
14.4% |
|||||
|
Results at 6 months are provided with italic numbers. #1: Early generation THV#1: SAPIEN XT, CoreValve. #2: Newer generation THV#2: SAPIEN 3, Evolut R, JenaValve, Direct Flow, J-Valve, Engager, Portico, Acurate, Lotus. a: Mild or greater PVR. b: Logistic EuroSCORE or EuroSCORE II. *: Procedural success was defined as the successful transcatheter implantation of a functioning aortic valve according to Valve Academic Research Consortium criteria. +: Procedural success was defined as the successful transcatheter implantation of a functioning aortic valve according to Valve Academic Research Consortium 2 criteria. †: Procedural success was defined as the successful transcatheter implantation of a functioning aortic valve according to Valve Academic Research Consortium 3 criteria. PVR = paravalvular regurgitation; STS-PROM = Society of Thoracic Surgeons Predicted Risk of Mortality; THV = transcatheter heart valve. |
||||||||||
Several studies have reported that TAVI using THV devices off-label for the treatment of pure severe AR is associated with a higher risk of procedural complications (valve embolization and need for second valve implantation) and device failure, . The PANTHEON International Project evaluated the performance of new-generation THV in patients treated for pure native severe AR. The study included 201 patients who underwent TAVI with currently available devices. The overall technical and device success rates (according to the VARC-3 criteria) were 83.6% and 76.1%, respectively, and the procedural failure were predominantly due to device embolization (12.4%) and residual moderate or greater AR (9.6%). Of note, there were no differences in the complication rates between the balloon-expandable and self-expanding devices.
To overcome the technical challenges, dedicated TAVI systems have been designed. The JenaValve (JenaValve Technology) and J-Valve (JC Medical) are dedicated self-expanding valve systems for the treatment of pure AR. Both THV are designed to provide active anchoring features in noncalcified aortic valve leaflets, via 3 anchoring clips in the JenaValve and 3 anchoring rings in the J-Valve system. Although earlier generation devices were delivered via transapical access, , both systems have been completely redesigned for transfemoral access in current generation of devices (Figure 33).
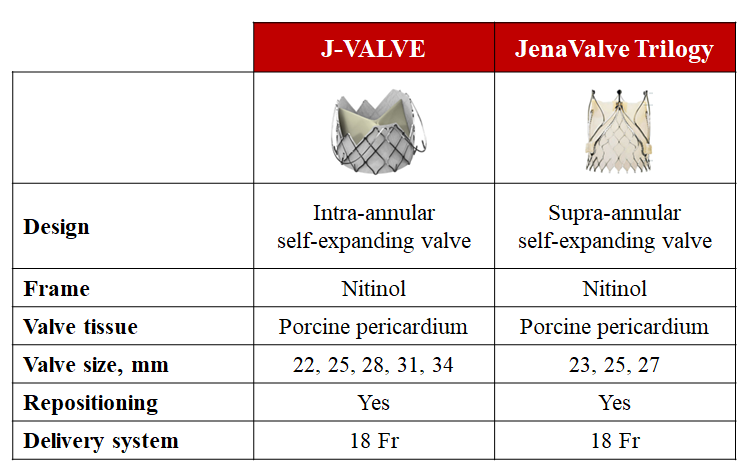
Dedicated transcatheter aortic valve implantation devices for the treatment of pure aortic regurgitation.
The latest iteration of the JenaValve, the JenaValve Trilogy, consists of a supra-annular porcine pericardial bioprosthetic valve with a nitinol frame. The unique feature of the Trilogy valve is the locator technology that ensures optimal positioning within the native aortic valve and secure anchoring by 3 radial locators that are positioned in each aortic cusp under fluoroscopy or transesophageal echocardiography guidance, ensuring commissural alignment and attachment to the cusps upon valve release. The frame has three large open cells, enabling coronary artery access after implantation. The prosthesis is available in 3 sizes (23 mm, 25 mm, and 27 mm), allowing treatment of annulus diameters up to 28.6 mm, while treatment of bicuspid valves is currently not recommended. A German multicentre study was the first to report the clinical outcomes of the JenaValve Trilogy. In the analysis of 58 patients who underwent TAVI with the JenaValve Trilogy for the treatment of pure AR, device success was achieved in 98% of patients, with no moderate or greater PVR, valve embolization, or conversion to surgery. At 30 days, 92% of the patients were in NYHA functional class I or II, and the 30-day mortality rate was 1.7%. The ALIGN-AR trial is the first, prospective, multicentre, single-arm pivotal study of transfemoral TAVI with the JenaValve Trilogy in patients with symptomatic moderate-to-severe or severe AR at high surgical risk. Anatomical exclusion criteria included bicuspid aortic valve morphology, aortic annulus perimeter derived diameter of <21.0 mm or >28.6 mm or perimeter <66 mm or >90 mm, aortic annulus angulation >70 degree, straight length of ascending aorta of <55 mm, and significant disease of ascending aorta (ascending aortic aneurysm defined as maximal luminal diameter of 50 mm or greater or atheroma including if thick [>5 mm], protruding or ulcerated). The primary safety endpoint was all-cause mortality, any stroke, major vascular complication, life-threatening or major bleeding, new pacemaker, acute kidney injury, valve dysfunction and surgery or intervention related to the device at 30 days, and the primary efficacy endpoint was all-cause mortality at 1 year. This study included 180 patients (mean age was 75 years, 47% were female, and mean STS-PROM was 4.1%). Technical success was achieved in 171 (95%) patients, and 30-day all-cause mortality, any stroke, and moderate or greater residual AR occurred in 2.2%, 2.2%, and 0.6%, respectively. The primary safety endpoint was met in 48 patients with a prespecified non-inferiority margin (26.7%, 97.5% CI 19.2-34.0, Pnon-inferiority <0.0001), with new pacemaker implantation in 36 (24%) patients. At 1 year, the primary efficacy endpoint of all-cause mortality was low (7.8%, 97.5% CI 3.3-12.3, Pnon-inferiority <0.0001) and the JenaValve Trilogy demonstrated significant and sustained improvement in functional status and patient-reported outcomes (6-min walk test: from 262.7 m at baseline to 312.5 m at 1 year, P = 0.004; and KCCQ overall score: from 55.3% at baseline to 77.6% at 1 year, P <0.0001). At 2 years, all-cause and cardiovascular death and all-cause stroke occurred in 15.4%, 7.4%, and 6.4% of patients, respectively, and the bioprosthetic haemodynamic properties and improved patient health status were sustained. In addition, echocardiography demonstrated favourable LV remodeling that was sustained over the 2-year follow-up period. Based on the favourable results of the Trilogy System, a randomized clinical trial is currently ongoing to demonstrate non-inferiority of the Trilogy THV System compared to SAVR for the treatment of patients with clinically significant native AR (ARTIST: Aortic Regurgitation Trial Investigating Surgery Versus Trilogy [NCT06608823]).
The J-Valve consists of 3 bovine pericardial leaflets in a self-expanding, low-profile nitinol frame with 3 U-shaped “anchor rings” and has a similar anchoring concept to that used for the Trilogy prosthesis. The prosthesis is available in 5 sizes (22, 25, 28, 31, and 34 mm) to treat a wide range of anatomies, with an annular diameter of up to 33.1 mm. A compassionate use experience in North America was reported in which 27 patients (median age 81 years, 81% at high surgical risk, 96% in NYHA functional class III or IV) with native valve AR were treated with the J-Valve (2018-2022). Although adverse clinical outcomes were rare at 30 days (1 death, 1 stroke, 3 permanent pacemaker implantations, and no moderate or greater AR), procedural success defined as the J-Valve delivered to the intended location without the need for surgical conversion or a second THV was achieved in 81% (22 of 27 cases), which led to a redesign of the attachment points and bonding methods. After the valve design was modified, the compassionate use experience was successfully continued without surgical conversion or the need for a second THV. The J-Valve TF Early Feasibility Study (JVTF EFS) prospectively evaluated the safety and efficacy of the J-Valve in patients with severe symptomatic AR, high surgical risk, and suitable anatomy for transfemoral access. The study included 15 high-risk patients (mean age 80 years, 73% male, STS-PROM 5.5%). The procedural success rate was 93.5% (14 of 15 cases): one patient required conversion to surgery due to an inability to release the anchor rings after a successful valve deployment, secondary to extreme aortic tortuosity. At 30 days, there were no cardiovascular deaths, strokes, device-related interventions, or new permanent pacemakers. Echocardiographic outcomes showed no or trace residual AR in all patients, and a paired analysis showed an evidence of left ventricular remodeling. The J-Valve Transfemoral Pivotal Study (JOURNEY) is currently ongoing to evaluate the safety and efficacy of the J-Valve Transfemoral System in patients with symptomatic, severe (grade 3 or 4) native AR and AR-dominant mixed aortic valve disease (NCT06455787).
Horacio Medina de Chazal, Ali Zgheib, Angelo Quagliana, Michael Chetrit, Jean Buithieu, Giuseppe Martucci, Marco Spaziano, Ali Abualsaud, Ole de Baker, Laurence Campens, Pascal Theriault-Lauzier, Jere...
Updated on November 23, 2022
Taishi Okuno, Daijiro Tomii, Thomas Pilgrim, Stephan Windecker
Updated on May 13, 2021
Horacio Medina de Chazal, Ali Zgheib, Angelo Quagliana, Michael Chetrit, Jean Buithieu, Giuseppe Martucci, Marco Spaziano, Ali Abualsaud, Ole de Baker, Laurence Campens, Pascal Theriault-Lauzier, Jere...
Updated on November 17, 2023